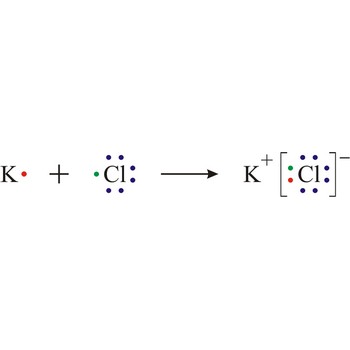ion exchange → ionska izmjena
Ion exchange is a process involving the adsorption of one or several ionic species accompanied by the simultaneous desorption (displacement) of one or more other ionic species.
ion exchanger → ionski izmjenjivač
Ion-exchanger is a solid or liquid material containing ions that are exchangeable with other ions with a like charge that are present in a solution in which the material is insoluble. Ion-exchange resins consist of various copolymers having a cross-linked three-dimensional structure to which ionic groups have been attached.
complete ionic equation → potpuna ionska jednadžba
Complete ionic equation is a balanced equation that describes a reaction occurring in a solution, in which all strong electrolytes are written as dissociated ions.
ionic bond → ionska veza
Ionic bond is a strong force of attraction holding atoms together in a molecule or crystal. Typically chemical bonds have energies of about 100 kJ mol-1. Ionic bond is a bond at which one of the participants, during the procedure of bonding, gives away its unpaired electrons to another atom so that both can achieve electron arrangement of the closest noble gas. In order to form an ionic bond one of the atoms must cross to the positively charged ion by losing certain number of electrons and the other atom must receive those electrons and cross to the negatively charged ion.
ionic strength → ionska jakost otopine
Ionic strength (μ or I) is a measure of the total concentration of ions in a solution, defined by
where zi is the charge of ionic species i and ci is its concentration.
anion exchange → anionski izmjenjivač
An anionic resin has negative ions built into its structure and therefore exchanges positive ions. In an anion exchange, the side groups are ionised basic groups, such as (-NH2, -NRH, -NR2, -NR3+) to which anions OH- are attached. The exchange reaction is one in which different anions in the solution displace the OH- from the solid.
cation exchange → kationski izmjenjivač
Cation exchange is a cationic resin has positive ions built into its structure and therefore exchanges negative ions. In the cation exchange, the side groups are ionised acidic groups, such as (-SO3H, -COOH, -OH) to which cations H+ are attached. The exchange reaction is one in which different cations in the solution displace the H+ from the solid.
ionic conductor → ionski vodič
Ionic conductor is a material that conducts electricity with ions as charge carriers.
ion-product constant → ionski produkt vode
The ion-product constant. For the reaction:
the equilibrium expression would be:
Note that all pure liquid terms are omitted, hence H2O does not appear in the denominator. At 25 °C
Citing this page:
Generalic, Eni. "Ionska izmjena." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table