lactose → laktoza
Lactose (milk sugar) is a disaccharide comprising one glucose molecule linked to a galactose molecule by an β(1→4)-glycosidic linkage. Lactose has a beta acetal. Lactose is manufactured by the mammary gland and occurs only in milk (from 4 % to 7 %). Lactose intolerance is a common medical condition that results in diarrhea, abdominal pain, and flatulence and is caused by reduced or absent activity of enzyme lactase.
Like cellobiose and maltose, lactose is a reducing sugar. All reducing sugar undergo mutarotation in aqueous solution. The equilibrium mixture at 20 °C is composed of 62.7 % β-lactose (β-D-galactopyranosyl-(1→4)-β-D-glucopyranose) and 37.3 % α-lactose (β-D-galactopyranosyl-(1→4)-α-D-glucopyranose).
mutarotation → mutarotacija
Mutarotation is the change in optical rotation accompanying epimerization. In carbohydrate chemistry this term usually refers to epimerization at the hemiacetal carbon atom. In general α- and β-form are stable solids, but in solution they rapidly equilibrate. For example, D-glucose exists in an equilibrium mixture of 36 % α-D-glucopyranose and 64 % β-D-glucopyranose, with only a tiny fraction in the open-chain form. The equilibration occurs via the ring opening of the cyclic sugar at the anomeric center with the acyclic form as the intermediate. Mutarotation was discovered by French chemist Augustin-Pierre Dubrunfaut (1797-1881) in 1846.
monosaccharide → monosaharid
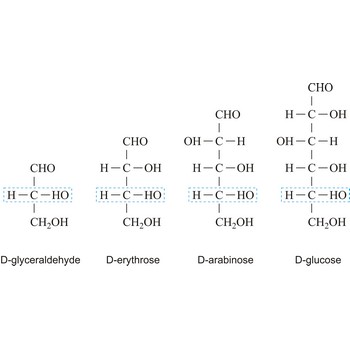
Monosaccharides are carbohydrates, with the general formula Cn(H2O)n, that cannot be decomposed to a simpler carbohydrates by hydrolysis.
Depending on whether the molecule contains an aldehyde group (-CHO) or a ketone group (-CO-) monosaccharide can be a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose). These aldehyde and ketone groups confer reduction properties on monosaccharides. They are also classified according to the number of carbon atoms they contain: trioses have three carbon atoms, tetroses four, pentoses five, hexoses six, heptoses seven, etc. These two systems of classification are often combined. For example, a six-carbon polyhydroxy aldehyde such as D-glucose is an aldohexose, whereas a six-carbon polyhydroxy ketone such as D-fructose is a ketohexose.
The notations D and L are used to describe the configurations of carbohydrates. In Fischer projections of monosaccharides, the carbonyl group is always placed on top (in the case of aldoses) or as close to the top as possible (in the case of ketoses). If the OH group attached to the bottom-most asymmetric carbon (the carbon that is second from the bottom) is on the right, then the compound is a D-sugar. If the OH group is on the left, then the compound is an L-sugar. Almost all sugars found in nature are D-sugars.
Monosaccharides can exist as either straight-chain or ring-shaped molecules. During the conversion from straight-chain form to cyclic form, the carbon atom containing the carbonyl oxygen, called the anomeric carbon, becomes a chiral center with two possible configurations (anomers), α and β. When the stereochemistry of the first carbon matches the stereochemistry of the last stereogenic center the sugar is the α-anomer when they are opposite the sugar is the β-anomer.
nucleic acid → nukleinska kiselina
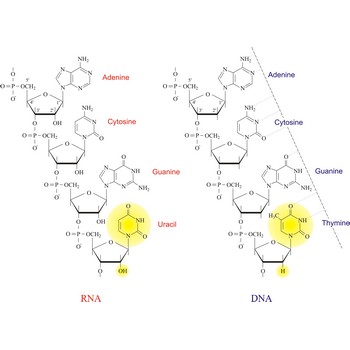
Nucleic acids are a complex, high-molecular-weight biochemical macromolecules composed of nucleotide chains that convey genetic information. The most common nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Each nucleic acid chain is composed of subunits called nucleotides, each containing a sugar, a phosphate group, and nitrogenous base. DNA was first discovered in 1869 by the Swiss biochemist Friedrich Miescher (1844-1895).
Both DNA and RNA contain the two major purine bases adenine (A) and guanine (G) and one of the major pyrimidines, cytosine (C). Of the other two pyrimidines, thymine (T) is found in DNA and uracil (U) is found in RNA. There are two major pentoses in nucleic acids:2'-deoxy-D-ribose in DNA and D-ribose in RNA.
Nucleotides are linked together in both DNA and RNA in a polymeric fashion via covalent bonds. These bonds exist through phosphate-group bridges in which the 5' hydroxyl group of one nucleotide unit is joined to the 3' hydroxyl group of the next nucleotide. RNA is usually a single-stranded molecule, whereas DNA is usually double-stranded.
Newton’s gravitational law → Newtonov zakon gravitacije
Every object in the universe attracts every other object with a force (gravitational force FG) directed along the line through centres of the two objects that is proportional to the product of their masses and inversely proportional to the square of the distance between them.
m1 and m2 are masses of the two objects and r is the distance between them. G is universal constant of gravitation, which equals 6.67•10-26 N m2 kg-2. Strictly speaking, this law applies only to objects that can be considered pointlike object. Otherwise, the force has to be found by integrating the forces between various mass elements.
It is more properly to express Newton’s gravitational law by vector equation:
in which r1 and r2 are position vectors of masses m1 and m2.
Gravitational forces act on distance. Newton’s gravitational law is derived from Kepler’s law for planetary motion, using a physical assumption considering Sun as the centre and the source of gravitational force.
Additionally, every object moves in the direction of the force acting on it, with acceleration that is inversely proportional to the mass of object. For bodies on the surface of Earth, the distance r in gravitational law formula is practically equal to the Earth radius, RE. If the mass of the body on Earth surface is m and the mass of earth is ME, the gravitational force acting on that body can be expressed as:
where g is gravitational acceleration which is, although dependent on geographical latitude, usually considered as constant equal to 9.81 m s-2.
nucleotide → nukleotid
Nucleotides are the components that made up nucleic acids. They have three major components: the first component is a nitrogenous base, which is derivative of one of two parent compounds, pyrimidine or purine; the second is a pentose, or five carbon sugar group; the third is a unit of phosphate. Each group of three nucleotides in a gene is known as a codon. Whenever the phosphate group is not present, a nucleotide becomes a nucleoside.
polysaccharide → polisaharid
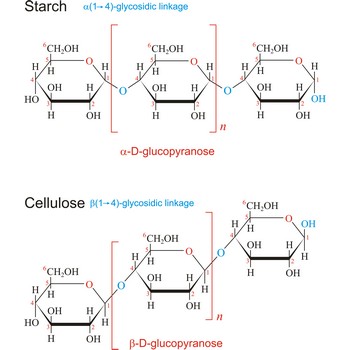
Polysaccharides are compounds consisting of a large number of simple sugars (monosaccharides) linked together by glycosidic bonds. When polysaccharides are composed of a single monosaccharide building block, they are termed homopolysaccharides. Heteropolysaccharides contain two or more different types of monosaccharide. Polysaccharides may have molecular weights of up to several million and are often highly branched. Since they have only the one free anomeric -OH group at the end of a very long chain, polysaccharides aren’t reducing sugars and don’t show noticeable mutarotation. The most common polysaccharides are cellulose, starch, and glycogen.
refractometer → refraktometar
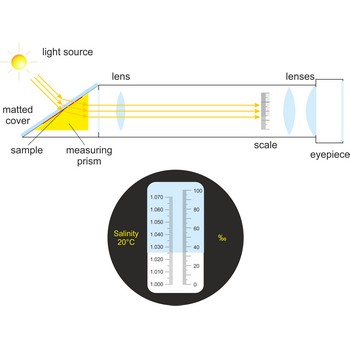
Refractometer is an optical device used from measurement of refractive index. A refractometer takes advantage of the fact that light bends as it passes through different materials. It can be used to measure the salinity of water or the amount of sugar in fresh grapes. Refractometers are available with or without automatic temperature compensation (ATC).
When using a conventional saltwater refractometer, a sample is placed on an optical prism in the sample window. As light shines through the sample, it is bent according to the salinity of the water, and casts a shadow on the scale that is visible through the eyepiece. Salinity is read directly through the eyepiece.
Citing this page:
Generalic, Eni. "Invert sugar." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table