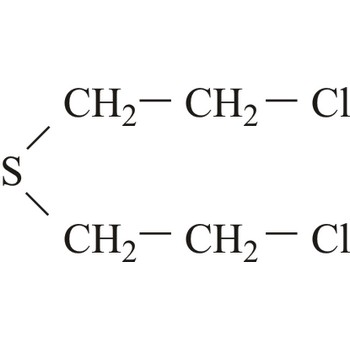lead-acid battery → olovni akumulator
Lead-acid battery is a electrical storage device that uses a reversible chemical reaction to store energy. It was invented in 1859 by French physicist Gaston Planté. Lead-acid batteries are composed of a lead(IV) oxide cathode, a sponge metallic lead anode and a sulphuric acid solution electrolyte.
In charging, the electrical energy supplied to the battery is changed to chemical energy and stored. The chemical reaction during recharge is normally written:
In discharging, the chemical energy stored in the battery is changed to electrical energy. During discharge, lead sulfate (PbSO4) is formed on both the positive and negative plates. The chemical reaction during discharge is normally written:
Lead acid batteries are low cost, robust, tolerant to abuse, tried and tested. For higher power applications with intermittent loads however, they are generally too big and heavy and they suffer from a shorter cycle life.
mass-energy equivalence → ekvivalencija mase i energije
In the special theory of relativity Einstein demonstrated that neither mass nor energy were conserved separately, but that they could be traded one for the other and only the total "mass-energy" was conserved. The relationship between the mass and the energy is contained in what is probably the most famous equation in science,
Where m is the mass of the object and c is the velocity of light. Cockcroft and Walton (1932) are routinely credited with the first experimental verification of mass-energy equivalence.
microscope → mikroskop
Microscope is an instrument that produces enlarged images of small objects. The optical microscopes (light microscope) use visible light and a system of lenses to magnify images. Typical magnification of a light microscope is up to 1500× ("1500 times")with a theoretical resolution limit of around 200 nm. Instead of using light, electron microscopes transmit a beam of electrons through, or onto the surface of, a specimen. An electron beam has a much shorter wavelength than does light, and can reveal structures as small as 2 nm.
mole → mol
Mole (mol) is the SI base unit of amount of substance.
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kg of carbon 12.
When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles. In this definition, it is understood that the carbon 12 atoms are unbound, at rest and in their ground state.
mustard agent → plikavac
Mustard agents are usually classified as blistering agents owing to the similarity of the wounds caused by these substances resembling burns and blisters. However, since mustard agents also cause severe damage to the eyes, respiratory system and internal organs, they should preferably be described as blistering and tissue-injuring agents. Normal mustard agent (yperite), 1,1-thio-bis-[2-chloroethane], reacts with a large number of biological molecules. The effect of mustard agent is delayed and the first symptoms do not occur between 2-24 hours after exposure. At room temperature, mustard agent is a liquid with low volatility and is very stable during storage.
Nernst’s electrode potential equation → Nernstova jednadžba za elektrodni potencijal
For general reaction of some redox system
dependence of electrode potential of redox system upon activity of oxidised and reduced form in solution is described in Nernst’s equation for electrode potential:
where E = to electrode potential of redox system
E° = standard electrode potential of redox system
R = universal gas constant
T = thermodymical temperature
F = Faraday’s constant
z = number of electrons exchanged in redox reaction
aO = activity of oxidised form
aR = activity of reduced form
n = stechiometrical coefficient of oxidised form
m = stechiometrical coefficient of reduced form
nerve poison → živčani bojni otrov
Nerve poison (nerve gas, agents) have had an entirely dominant role since the Second World War. Nerve poisons acquired their name because they affect the transmission of nerve impulses in the nervous system. All nerve poisons belong chemically to the group of organo-phosphorus compounds. They are stable and easily dispersed, highly toxic and have rapid effects both when absorbed through the skin and via respiration. Nerve poisons can be manufactured by means of fairly simple chemical techniques. The raw materials are inexpensive and generally readily available.
The most important nerve agents included in modern chemical weapons arsenals are:
| Tabun | (o-ethyl dimethylamidophosphorylcyanide) |
| Sarin | (isopropyl methylphosphonofluoridate) |
| Soman | (pinacolyl methylphosphonofluoridate) |
| GF | (cyclohexyl methylphosphonofluoridate) |
| VX | (o-ethyl S-diisopropylaminomethyl methylphosphonothiolate) |
Nerve poisons are colorless, odorless, tasteless liquids of low volatility. Antidotes are atropine sulfate and pralidoxime iodide.
noble gas → plemeniti plin
Noble gas refers to any element of the group of six elements in group 18 of the periodic table. They are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Unlike most elements, the noble gases are monoatomic. The atoms have stable configurations of electrons. Therefore, under normal conditions they do not form compounds with other elements.
They were generally called inert gases until about 1962 when xenon tetrafluoride, XeF4, was produced in the laboratory. This was the first report of a stable compound of a noble gas with another single element.
non-metal → nemetal
Non-metals are defined as elements that are not metals.
Their physical properties generally include:
- They are poor conductors.
- They are brittle, not ductile in their solid state.
- They show no metallic lustre.
- They may be transparent or translucent.
- They have low density.
- They form molecules which consists of atoms covalently bonded; the noble gases are monoatomic.
Their chemical properties are generally:
- They usually have four to eight valence electrons.
- They have high electron affinities (except the noble gases)
- They are good oxidising agents (except the noble gases)
- They have hydroxides which are acidic (except the noble gases)
- They are electronegative.
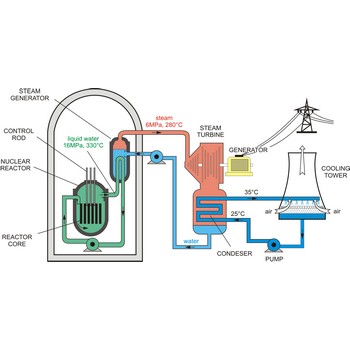
nuclear reactor → nuklearni reaktor
Nuclear reactor is an assembly of fissionable material (uranium-235 or plutonium-239) designed to produce a sustained and controllable chain reaction for the generation of electric power.
The essential components of a nuclear reactor are:
- The core, metal rods containing enough fissionable material to maintain a chain reaction at the necessary power level (as much as 50 t of uranium may be required).
- A source of neutrons to initiate the reaction (such as a mixture of polonium and beryllium)
- A moderator to reduce the energy of fast neutrons for more efficient fission (material such as graphite, beryllium, heavy water, and light water are used)
- A coolant to remove the fission-generated heat (water, sodium, helium, and nitrogen may be used)
- A control system such as rods of boron or cadmium that have high capture cross sections (to absorb neutrons)
- Adequate shielding, remote-control equipment, and appropriate instrumentation are essential for personnel safety and efficient operation.
Citing this page:
Generalic, Eni. "Inercijski referentni sustavi." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table