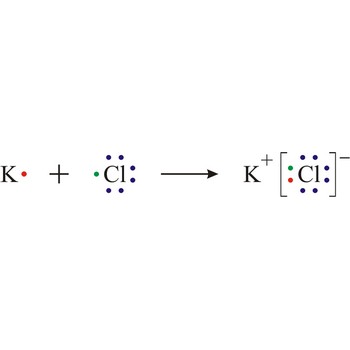global warming → globalno zatopljenje
Global warming or greenhouse effect is an effect occurring in the atmosphere because of the presence of certain gases (greenhouse gases) that absorb infrared radiation. Light and ultraviolet radiation from the sun is able to penetrate the atmosphere and warm the Earth’s surface. This energy is re-radiated as infrared radiation which because of its longer wavelength, is absorbed by such substances as carbon dioxide. The overall effect is that the average temperature of the Earth and its atmosphere is increasing (so-called global Warming). The effect is similar to that occurring in a greenhouse, where light and long-wavelength ultraviolet radiation can pass through the glass into greenhouse but the infrared radiation is absorbed by the glass and part of it is re-radiated into the greenhouse.
The greenhouse effect is seen as a major environmental hazard. Average increases in temperature could change weather patterns and agricultural output. It might also lead to melting of the polar ice caps and a corresponding rise in sea level. Carbon dioxide, from fossil-fuel power stations and car exhausts, is the main greenhouse gas. Other contributory pollutants are nitrogen oxides, ozone, methane, and chloroflourocarbons.
Graham’s law → Grahamov zakon
Graham’s law is the rates at whish gases diffuse are inversely proportional to the square roots of their densities. This principle is made use of in the diffusion method of separating isotopes. The law was formulated in 1829 by British chemist Thomas Graham (1805-1869).
groups in periodic system of elements → skupine periodnog sustava
Periodic system of elements is divided into 18 groups of chemical elements. Elements belonging to the same group have a same number of valence electrons and similar chemical properties. Elements of main groups are in 1., 2., and in groups 13. to 18. Different groups of elements can be named according to the first element in the group (elements of boron group, elements of carbon group), or they have some special names (noble gases, halogenic elements, halyde elements, earthalkali and alkali metals).
Haber process → Haberov proces
Haber process is an industrial process for producing ammonia by reaction of nitrogen with hydrogen:
The reaction is reversible and exothermic, so that a high yield of ammonia is favoured by low temperature. However, the rate of reaction would be too slow for equilibrium to be reached at normal temperatures, so an optimum temperature of about 450 °C is used, with a catalyst of iron containing potassium aluminium oxide promoters. The higher the pressure the greater the yield, although there are technical difficulties in using very high pressures. A pressure of about 250 atmospheres is commonly employed. The removal of ammonia from the batch as soon as it is formed ensures that an equilibrium favouring product formation is maintained. The nitrogen is obtained from air. Formerly, the hydrogen was from water gas and the water-gas shift reaction (the Bosch process) but now the raw material (called synthesis gas) is obtained by steam reforming natural gas.
The process is of immense importance for the fixation of nitrogen for fertilisers and explosives. It was developed in 1908 by German chemist Fritz Haber (1868-1934) and was developed for industrial use by Carl Bosch (1874-1940), hence the alternative name Haber-Bosch process.
halogens → halogeni elementi
Halogens are the elements fluorine (F) chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They are non-metals, and make up part of the 17 group in the periodic table. Compounds of these elements are called halogenides or halides.
The halogens all have a strong unpleasant odour and will burn flesh. They do not dissolve well in water. The five elements are strongly electronegative. They are oxidising agents, with fluorine being the strongest and astatine being the weakest. They react with most metals and many non-metals.
Halogens form molecules which consist of atoms covalently bonded. With increasing atomic weight there is a gradation in physical properties. For example: Fluorine is a pale green gas of low density. Chlorine is a greenish-yellow gas 1.892 times as dense as fluorine. Bromine is a deep reddish-brown liquid which is three times as dense as water. Iodine is a grayish-black crystalline solid with a metallic appearance. And astatine is a solid with properties which indicate that it is somewhat metallic in character.
helium → helij
Helium was discovered by Pierre Jules César Janssen (France) and Sir William Ramsay (Scotland) in 1868. The origin of the name comes from the Greek word helios meaning sun. It is light, odourless, colourless inert gas. Second most abundant element in the universe. Helium is found in natural gas deposits from wells in Texas, Oklahoma and Kansas. Used in balloons, deep sea diving and welding. Also used in very low temperature research.
Henry’s law → Henryjev zakon
Henry’s law was discovered in 1801 by the British chemist William Henry (1775-1836). At a constant temperature the mass of gas dissolved in a liquid at equilibrium is proportional to the partial pressure of the gas. It applies only to gases that do not react with the solvent.
where pi is the partial pressure of component i above the solution, xi is its mole fraction in the solution, and Kx is the Henry’s law constant (a characteristic of the given gas and solvent, as well as the temperature).
hydrogen → vodik
Hydrogen was discovered by Sir Henry Cavendish (England) in 1766. The origin of the name comes from the Greek words hydro and genes meaning water and generate. It is colourless, odourless gas, burns and forms explosive mixtures in air. Reacts violently with oxidants. Hydrogen is the most abundant element in the universe. Commercial quantities of hydrogen are produced by reacting superheated steam with methane or carbon. In lab work from reaction of metals with acid solutions or electrolysis. Most hydrogen is used in the production of ammonia and in metal refining. Also used as fuel in rockets. Its two heavier isotopes (deuterium and tritium) used respectively for nuclear fusion.
ionic bond → ionska veza
Ionic bond is a strong force of attraction holding atoms together in a molecule or crystal. Typically chemical bonds have energies of about 100 kJ mol-1. Ionic bond is a bond at which one of the participants, during the procedure of bonding, gives away its unpaired electrons to another atom so that both can achieve electron arrangement of the closest noble gas. In order to form an ionic bond one of the atoms must cross to the positively charged ion by losing certain number of electrons and the other atom must receive those electrons and cross to the negatively charged ion.
Joule-Thomson coefficient → Joule-Thompsonov koeficijent
Joule-Thomson coefficient (μ) is a parameter which describes the temperature change when a gas expands adiabatically through a nozzle from a high pressure to a low pressure region. It is defined by
where H is enthalpy.
Citing this page:
Generalic, Eni. "Idealni plin." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table