Fischer-Tropsch process → Fischer-Tropschov postupak
Fischer-Tropsch process is an industrial method of making hydrocarbon fuels from carbon monoxide and hydrogen. The process was introduced in 1933. and used by Germany in World War II. to produce motor fuel. Hydrogen and carbon monoxide are mixed in the ratio 2:1 (water gas was used with added hydrogen) and passed at 200 °C over a nickel or cobalt catalyst. The resulting hydrocarbon mixture can be separated into a higher-boiling fraction for Diesel engines and a lower-boiling petrol fraction. The petrol fraction contains a high proportion of straight-chain hydrocarbons and has to be reformed for use in motor fuel. Alcohols, aldehydes, and ketones are also present. The process is also used in the manufacture of SNG from coal. It is named after the German chemist Franz Fischer (1852-1932) and the Czech Hans Tropsch (1839-1935).
Frasch proces → Fraschov postupak
Frasch proces is a method of obtaining sulphur from underground deposits using a tube consisting of three concentric pipes. Superheated steam is passed down the outer pipe to melt the sulphur, which is forced up through the middle pipe by compressed air fed through the inner tube. The steam in the outer casing keeps the sulphur molten in the pipe. It was named after the German-born American chemist Herman Frasch (1851-1914).
gasoline → motorni benzin
Gasoline is a complex mixture of volatile hydrocarbons that may have between 5 to 12 carbons. The major components are branched-chain paraffins, cycloparaffins, and aromatics. Gasoline is most often produced by the fractional distillation of crude oil as the fraction of hydrocarbons in petroleum boiling between 30 °C and 200 °C. The quality of a fuel is measured with its octane number. Octane number is the measure of the resistance of gasoline against detonation or preignition of the fuel in the engine. The higher the octane number, the more compression the fuel can withstand before detonating. The octane number is determined by comparing the characteristics of a gasoline to isooctane with good knocking properties (octane number of 100) and heptane with bad (octane number of 0).
Gauss’ law for electrostatics → Gaussov zakon za elektrostatiku
Gauss’ law describes the relation between charge and electric field in static situations, so it is equivalent to Coulomb’s law, which can be derived from Gauss’ law. Gauss’ law states that the net flux of electric field, Φ, through an imaginary closed surface, S, - a Gaussian surface - is equal to the net charge, q, inside that closed surface:
where electric flux Φ through Gaussian surface is given by:
ε0 is the permittivity constant and dS is a surface element.
Gibbs phase rule → Gibbsov zakon faza
Gibbs phase rule is the relationship used to determine the number of state variables, usually chosen from among temperature, pressure, and species composition in each phase, which must be specified to fix the thermodynamic state of a system in equilibrium:
where C is the number of components in a mixture, P is the number of phases, and F is the degrees of freedom, i.e., the number of intensive variables that can be changed independently without affecting the number of phases.
global warming → globalno zatopljenje
Global warming or greenhouse effect is an effect occurring in the atmosphere because of the presence of certain gases (greenhouse gases) that absorb infrared radiation. Light and ultraviolet radiation from the sun is able to penetrate the atmosphere and warm the Earth’s surface. This energy is re-radiated as infrared radiation which because of its longer wavelength, is absorbed by such substances as carbon dioxide. The overall effect is that the average temperature of the Earth and its atmosphere is increasing (so-called global Warming). The effect is similar to that occurring in a greenhouse, where light and long-wavelength ultraviolet radiation can pass through the glass into greenhouse but the infrared radiation is absorbed by the glass and part of it is re-radiated into the greenhouse.
The greenhouse effect is seen as a major environmental hazard. Average increases in temperature could change weather patterns and agricultural output. It might also lead to melting of the polar ice caps and a corresponding rise in sea level. Carbon dioxide, from fossil-fuel power stations and car exhausts, is the main greenhouse gas. Other contributory pollutants are nitrogen oxides, ozone, methane, and chloroflourocarbons.
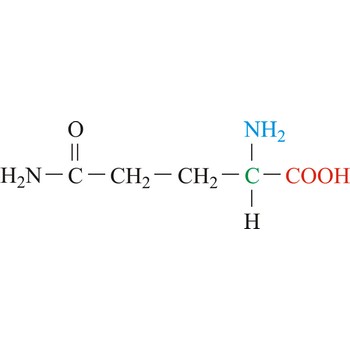
glutamic acid → glutaminska kiselina
Glutamic acid is an electrically charged amino acids. It is one of the two amino acids that contain a carboxylic acid group in its side chains. These acids play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins. Glutamic acid is commonly referred to as glutamate, because its carboxylic acid side chain will be deprotonated and thus negatively charged in its anionic form at physiological pH. Glutamic acid is referred to as a non-essential amino acid because a healthy human can synthesize all the glutamic acid needed for normal body function from other amino acids.
- Abbreviations: Glu, E
- IUPAC name: 2-aminopentanedioic acid
- Molecular formula: C5H9NO4
- Molecular weight: 147.13 g/mol
glucose → glukoza
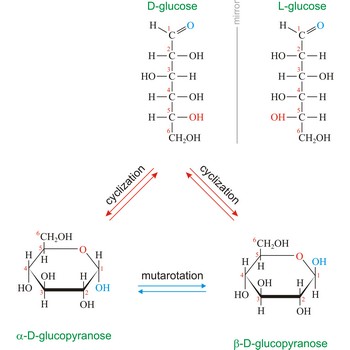
Glucose (grape sugar, blood sugar), C6H12O6, is an aldohexose (a monosaccharide sugar having six carbon atoms and an aldehyde group). An older common name for glucose is dextrose, after its dextrorotatory property of rotating plane polarized light to the right. Glucose in free (in sweet fruits and honey) or combined form (sucrose, starch, cellulose, glycogen) is is probably the most abundant organic compound in nature. During the photosynthesis process, plants use energy from the sun, water from the soil and carbon dioxide gas from the air to make glucose. In cellular respiration, glucose is ultimately broken down to yield carbon dioxide and water, and the energy from this process is stored as ATP molecules (36 molecules of ATP across all processes).
Naturally occurring glucose is D isomers (OH group on the stereogenic carbon farthest from the aldehyde group, C-5, is to the right in the Fischer projection). Although often displayed as an open chain structure, glucose and most common sugars exist as ring structures. In the α form, the hydroxyl group attached to C-1 and the CH2OH attached to C-5 are located on opposite sides of the ring. β-glucose has these two groups on the same side of the ring. The full names for these two anomers of glucose are α-D-glucopyranose and β-D-glucopyranose.
glutamine → glutamin
Glutamine is neutral amino acids with polar side chains. It serves as an important carrier of ammonia and contributes it to the formation of urea and purines. Glutamine is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders. It is synthesized by the enzyme glutamine synthetase from glutamate and ammonia.
- Abbreviations: Gln, Q
- IUPAC name: 2,5-diamino-5-oxopentanoic acid
- Molecular formula: C5H10N2O3
- Molecular weight: 146.14 g/mol
glycine → glicin
Glycine is the smallest amino acid and is unique because it lacks a side chain. This gives it more conformational freedom than any other amino acid. Glycine is often found in turns and loops where other amino acids would be sterically unacceptable. Although it is formally nonpolar, it’s very small side chain makes no real contribution to hydrophobic interactions. Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acid serine.
- Abbreviations: Gly, G
- IUPAC name: 2-aminoacetic acid
- Molecular formula: C2H5NO2
- Molecular weight: 75.07 g/mol
Citing this page:
Generalic, Eni. "Hera chan tvb." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table