Le Chatelier’s principle → Le Chatelierov princip
The idea that a system at equilibrium will respond to a stress placed upon it in such a manner as to partially offset that stress. The principle was first stated in 1888 by the French physical chemist Henri Le Chatelier (1850-1936).
salt fog chambers → slana komora
Salt fog chambers are designed for corrosive atmosphere testing. The samples being tested are inserted into the chamber and then the salt-containing solution is sprayed as a very fine fog mist over the samples. The temperature within the chamber is maintained constant (usually 35 °C). These test chambers are constructed of non-corrosive materials.
lateral chain → postranični lanac
Lateral chain is a shorter chain of hydrocarbons which is connected to the main chain of hydrocarbon.
Wilson’s chamber → Wilsonova komora
Wilson’s chamber is used for detection of radioactive radiation. Wilson’s chamber has a glass cylinder filled with air that has been saturated with water vapour. Radioactive radiation in its way ionises molecules of gas which then function as centres on which water vapour condenses into very small drops, thereupon showing Tyndall’s effect, i.e. is they are visible as a bright trail.
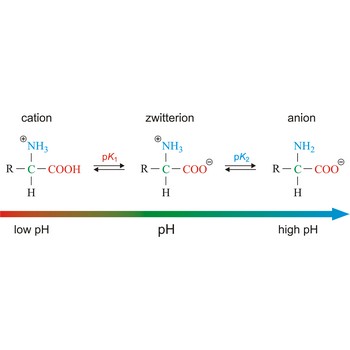
zwitterion → dipolarni ion
Zwitterion, also known as inner salt or dipolar ion, is an ion with a positive and a negative electrical charge at different locations within a molecule. As the molecule contains two opposite charges, it is electrically neutral. The term zwitterion is derived from the German word zwitter, meaning a hybrid, hermaphrodite. Zwitterions can be formed from compounds that contain both acid groups and base groups in their molecules (ampholytes).
All of the common amino acids found in proteins are ampholytes because they contain a carboxyl group (-COOH) that acts as an acid and an amino group (-NH2) that acts as a base. In the solid state, amino acids exist in the dipolar or zwitterion form. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (H+) ion, and the amino acid becomes positively charged. If base is added, ion removal of the H+ ion from the amino group of the zwitterion produces a negatively charged amino acid.
Charles’ law → Charlesov zakon
The volume of a fixed mass of gas at a constant pressure expand by the constant fraction of its volume at 0 °C. For each Celsius or kelvin degree its temperature is raised. For any ideal gas fraction it is approximately 1/273. This can be expressed by the equation
were V° is the volume at 0°C and V is its volume at t°C.
This is equivalent to the statement that the volume of a fixed mass of gas at a constant pressure is proportional to its thermodynamic temperature
This law also know as Gay-Lussac’s law.
An equation similar to the one given above applies to pressures for ideal gases:
Citing this page:
Generalic, Eni. "Hera chan tvb." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table