silver coulometer → srebrni kulometar
Silver coulometer consists of a platinum vessel which acts as a cathode and contains a solution of pure silver nitrate as an electrolyte (c(AgNO3) = 1 mol/L). A rod of pure silver enclosed in a porous pot acts as the anode. The current density at the anode should not exceed 0.2 Acm-2. After electrolysis, the electrolyte is taken out and the platinum vessel is washed, dried and weighed. The increase in the weight gives the amount of silver deposited (96500 C of electricity deposits 107.88 g of silver). From the mass of the silver deposited, the coulomb involved in the reaction can be calculated.
specific weight → specifična težina
Specific weight (γ) is defined as the ratio between the weight of a mass element, Δm, and the volume, ΔV, occupied by that element. As density (average) is defined as the ratio of a mass element and its volume, specific weight is equal to:
where g is gravitational acceleration.
supercritical fluid → superkritični fluid
Supercritical fluid is any substance above its critical temperature and critical pressure (see phase diagram). It shows unique properties that are different from those of either gases or liquids under standard conditions. A supercritical fluid has both the gaseous property of being able to penetrate anything, and the liquid property of being able to dissolve materials into their components. Solublity increases with increasing density (i.e. with increasing pressure). An example of this is naphthalene which is practically insoluble in low pressure carbon dioxide. At 100 bar the solubility is 10 g/L and at 200 bar it is 50 g/L. Rapid expansion of supercritical solutions leads to precipitation of a finely divided solid.
Tafel plot → Tafelov dijagram
Tafel plot is the graph of the logarithm of the current density j against the overpotential η in electrochemistry in the high overpotential limit. An electrode when polarised frequently yields a current potential relationship over a region which can be approximated by:
where η is change in open circuit potential, i is the current density, B and i0 is constants. B is known as the Tafel Slope. If this behaviour is observed a plot of the semilogarithmic components is known as the Tafel line and the diagram is called the Tafel diagram.
titanium → titanij
Titanium was discovered by William Gregor (England) in 1791. Named after the Titans, the sons of the Earth goddess in Greek mythology. It is shiny, dark-grey metal. Powdered form burns in air. Exposed surfaces form oxide coating. It can be highly polished and is relatively immune to tarnishing. Unreactive with alkali and most acids. Titanium usually occurs in the minerals ilmenite (FeTiO3), rutile (TiO2) and iron ores. Pure metal produced by heating TiO2 with C and Cl2 to produce TiCl4 then heated with Mg gas in Ar atmosphere. Since it is strong and resists acids it is used in many alloys. Titanium dioxide (TiO2), a white pigment that covers surfaces very well, is used in paint, rubber, paper and many others.
transition metal → prijelazni element
This group of metals is distinguished from other metals not by their physical properties, but by their electronic structure. Transition metals are elements characterized by a partially filled d subshell. The First Transition Series comprises scandium (Sc), titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni) and copper (Cu). The Second and Third Transition Series include the lanthanides and actinides, respectively.
The transition metals are noted for their variability in oxidation state. Thus, manganese has two electrons in its outside shell and five electrons in the next shell down, and exhibits oxidation states of +1, +2, +3, +4, +5, +6, and +7.
They are also characterised by the fact that well into the series, going from left to right, the properties of the succeeding metals do not differ greatly from the preceding ones.
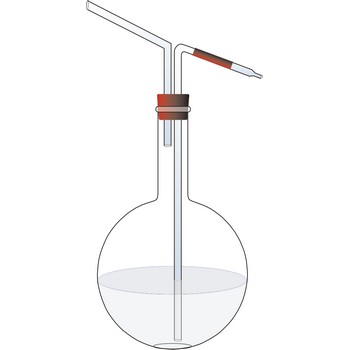
wash bottle → boca štrcaljka
Plastic wash bottle is a squeeze bottle made of low density polyethylene (LDPE) whose contents can be forced out through a narrow hole at the top by squeezing the bottle.
Glass wash bottle is a bottle fitted with two glass tubes pass through the cap, so that on blowing into one of the tubes a stream of water issuing from the other may be directed upon anything to be washed or rinsed, as a precipitate upon a filter.
Ziegler process → Zieglerov proces
Ziegler process is an industrial process for the manufacture of high-density polyethene using catalysts of titanium(IV) chloride (TiCl4) and aluminium alkyls (e.g. triethylaluminium, Al(C2H5)3). The process was introduced in 1953 by the German chemist Karl Ziegler (1898-1973). It allowed the manufacture of polythene at lower temperatures (about 60 °C) and pressures (about 1 atm) than used in the original process.
T-S diagram → T-S dijagram
The relationship between the temperature (T) and the salinity (S) of a seawater can be illustrated graphically on a T-S diagram, which is a simple, but powerful tool used in studies of seawater density, mixing, and circulation. In a T-S diagram, temperature is plotted along the vertical axis in degrees Celsius and salinity is measured along the horizontal axis in PSU (Practical Salinity Units). Seawater density is illustrated in the diagram by curved lines of constant density (isopycnals). Water tends to move horizontally throughout the deep ocean, moving along lines of equal density.
Chitosan → Kitozan
Chitosan is a linear polysaccharide composed of randomly distributed N-acetyl D-glucosamine and D-glucosamine units. It can be easily derived from partial deacetylation of natural polymer chitin. At a minimum deacetylization level of 60 % (amount of free amino groups in the polymer) it is considered to be chitosan. Thanks to the amino groups of D-glucosamine, chitosan can be protonated and turned into polycation, which is one of the sources of unique properties of chitosan as biopolymer, like aqueous solubility, antibacterial properties, biodegradability with non-toxic residues and biocompatibility.
Citing this page:
Generalic, Eni. "Gustoća." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table