fractional crystallisation → frakcijska kristalizacija
Fractional crystallisation is a method of separating a mixture of soluble solids by dissolving them in a suitable hot solvent and then lowering the temperature slowly. The least soluble component will crystallise out first, leaving the other components in the solution. By controlling the temperature, it is sometimes possible to remove each component in turn.
crystallisation → kristalizacija
Crystallisation is process in which the melted substance from a saturated solution turns into solid substance (crystal).
fractional distillation → frakcijska destilacija
Fractional distillation is a procedure in which liquids of close boiling points are separated. It is conducted in fraction or rectification columns in a way that vapour phase created by distillation is condensed and the condensate thus obtained is redistilled. The procedure is repeated several times. Vapour phase always contains more volatile component than the liquid phase, at top of the column vapour of clean volatile component gets out and at the bottom of the column liquid of nonvolatile component.
heat of crystallization → toplina kristalizacije
Heat of crystallization or enthalpy of crystallization is the heat evolved or absorbed when one mole of given substance crystallises from a saturated solution of the same substance.
argon → argon
Argon was discovered by Lord Raleigh and Sir William Ramsay (Scotland) in 1894. The origin of the name comes from the Greek word argos meaning inactive. It is colourless and odourless noble gas. Chemically inert. It is the third most abundant element in the earth’s atmosphere and makes up about 1 %. Argon is continuously released into the air by decay of radioactive potassium-40. Pure form is obtained from fractional distillation of liquid air. Used in lighting products. It is often used in filling incandescent light bulbs. Some is mixed with krypton in fluorescent lamps. Crystals in the semiconductor industry are grown in argon atmospheres.
azeotrope → azeotropi
Azeotrope is a mixture of two liquids that boils at constant composition, i.e. the composition of the vapour is the same as that of the liquid. Azeotropes occur because of deviations in Raoult’s law leading to a maximum or minimum in the boiling point - composition diagram. The composition of an azeotrope depends on the pressure.
chemical raw material → kemijske sirovine
Chemical raw material are petroleum fractions used for obtaining organic chemicals, those are mostly refined gas and petroleum or fraction parts of petrol.
coal tar → ugljeni katran
Coal tar is a material obtained from the destructive distillation of coal in the production of coal gas. The crude tar contains a large number of organic compounds (e.g. benzene, naphthalene, methylbenzene, etc.), which can be separated by fractional distillation.
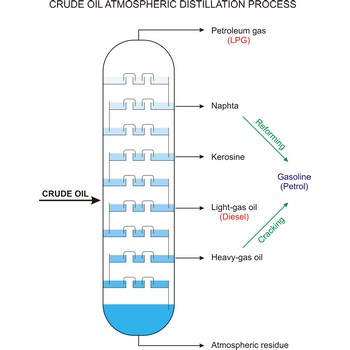
crude oil → sirova nafta
Crude oil (petroleum) is a fossil fuel formed from plant and animal remains many million of years ago. It is occasionally found in springs or pools but is usually drilled from wells beneath the earth’s surface. Crude oil is a mixture of hydrocarbons with small quantities of other chemicals such as sulphur, nitrogen and oxygen. Crude is the raw material which is refined into petrol, heating oil, jet fuel, propane, petrochemicals, and other products.
Citing this page:
Generalic, Eni. "Frakcijska kristalizacija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table