lattice energy → energija kristalne rešetke
Lattice energy is the energy per ion pair required to separate completely the ions in a crystal lattice at a temperature of absolute zero.
lattice constants → konstante kristalne rešetke
Lattice constants are parameters specifying the dimensions of a unit cell in a crystal lattice, specifically the lengths of the cell edges and the angles between them.
lattice → kristalna rešetka
Crystal lattice is a three-dimensional array of points that embodies the pattern of repetition in a crystalline solid. Don’t mix up atoms with lattice points: lattice points are infinitesimal points in space - atoms are physical objects.
activation energy → energija aktivacije
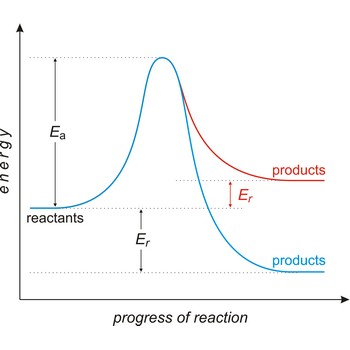
Activation energy (Ea) is the energy that must be added to a system in order for a process to occur, even though the process may already be thermodynamically possible. In chemical kinetics, the activation energy is the height of the potential barrier separating the products and reactants. It determines the temperature dependence on the reaction rate.
chemical energy → kemijska energija
Chemical energy is the energy stored in substances and transferred during chemical reaction.
free energy → slobodna energija
Free energy is an energy that is actually available to do useful work. A decrease in free energy accompanies any spontaneous process. Free energy does not change for systems that are at equilibrium.
Helmholz free energy → Helmholzova slobodna energija
Helmholz free energy (A) is a thermodynamic function defined by A = U - TS, where U is the internal energy, S the entropy, and T the thermodynamic temperature. For a reversible isothermal process ΔA represents the useful work available.
ionisation energy → energija ionizacije
Ionisation energy is the minimum energy required to remove an electron from an isolated atom or molecule (in its vibrational ground state) in the gaseous phase.
energy → energija
Energy (E, U) is the characteristic of a system that enables it to do work. Like work itself, it is measured in joules (J).
The internal energy of a body is the sum of the potential energy and the kinetic energy of its component atoms and molecules.
Potential energy is the energy stored in a body or system as a consequence of its position, shape, or state (this includes gravitation energy, electrical energy, nuclear energy, and chemical energy).
Kinetic energy is the energy of motion and is usually defined as the work that will be done by a body possessing the energy when it is brought to rest. For a body of mass m having a speed v, the kinetic energy is mv2/2. Kinetic energy is most clearly exhibited in gases, in which molecules have much greater freedom of motion than in liquids and solids.
In an isolated system energy can be transferred from one form to another but the total energy of the system remains constant.
undispersed energy → nedispergirana energija
Undispersed is the energy is energy of a small defined system.
Citing this page:
Generalic, Eni. "Energija kristalne rešetke." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table