alkaline earth metal → zemnoalkalijski metal
Alkali earth metal is a term that refers to six elements: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). These elements make up group 2 of the periodic table of elements. They all exhibit a single oxidation state, +2. They are all light and very reactive. Barium and radium are the most reactive and beryllium is the least.
To denote slightly soluble metal oxides chemists formerly used the term "earth". The oxides of barium, strontium, and calcium resemble alumina (Al2O3), a typical "earth", but form alkaline mixtures with water. For this reason barium, strontium, and calcium were called alkaline earth metals. This name has now been extended to include all of the elements of group 2.
alkanes → alkani
Alkanes (paraffins) are acyclic branched or unbranched hydrocarbons having the general formula CnH2n+2, and therefore consisting entirely of hydrogen atoms and saturated carbon atoms. In the systematic chemical nomenclature alkane names end in the suffix -ane. They form a homologous series (the alkane series) methane (CH4), ethane (C2H6), propane (C3H8), butane (C4H10), etc. The lower members of the series are gases; the high-molecular mass alkanes are waxy solid. Generaly the alkanes are fairly unreactive. They form haloalkanes with halogens when irradiated with ultraviolet radiation. Alkanes are present in natural gas and petroleum.
alkenes → alkeni
Alkenes are acyclic branched or unbranched hydrocarbons having one or more double carbon-carbon bonds in their molecules. In the systematic chemical nomenclature, alkene names end in the suffix -ene. The general formula is CnH(2n+2)-2x were x is the number of double bonds. Alkenes that have only one double bond form a homologous series: ethene (ethylene), CH2=CH2, propene, CH3CH2=CH2, etc. Alkenes typically undergo addition reactions to the double bond.
alkynes → alkini
Alkynes (acetylenes) are acyclic branched or unbranched hydrocarbons having one or more triple carbon-carbon bond. In the systematic chemical nomenclature alkyne names end in the suffix -yne. The general formula is CnH(2n+2)-4x were x is the number of triple bonds. Alkynes that have only one triple bond form a homologous series: ethyne (acetylene), CH≡CH, propyne, CH3CH≡CH, etc. Like alkenes, alkynes undergo addition reaction.
chemical kinetics → kemijska kinetika
Chemical kinetics is an area of chemistry that studies rates and mechanisms of chemical reactions.
chemiluminescence → kemiluminiscencija
Chemiluminescence is energy release in form of electromagnetic radiation during a chemical reaction.
complete ionic equation → potpuna ionska jednadžba
Complete ionic equation is a balanced equation that describes a reaction occurring in a solution, in which all strong electrolytes are written as dissociated ions.
allotrope → alotrop
Allotropes are the elements which exist in two or more different forms in the same physical state. Allotropes generally differ in physical properties and may also differ in chemical activity.
Diamond, graphite and fullerenes are three allotropes of the element carbon. Graphite is a soft, black, slippery substance; by contrast, diamond is one of the hardest substances known. The different properties of the allotropes arise from their chemical structures. Diamonds typically crystallize in the cubic crystal system and consist of tetrahedrally bonded carbon atoms. Graphite crystallizes in the hexagonal system. In the fullerenes, the carbon atoms taking the form of a hollow sphere, ellipsoid, or tube.
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C.
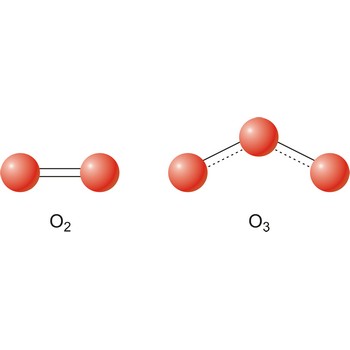
The term allotropes may also be used to refer to the molecular forms of an element. Ozone is a chemically active triatomic allotrope of the element oxygen.
allotropic modification → alotropska modifikacija
Different substances of the same elementary system are called allotropes or allotropic modifications. In the case of oxygen, there are two allotropic modifications: "normal" dioxygen (O2) and trioxygen (O3) or ozone.
Citing this page:
Generalic, Eni. "Elementarna reakcija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table