heat of hydration → toplina hidratacije
Heat of hydration or enthalpy of hydration of ions corresponds to the heat that is released by hydration of one mole of ions at a constant pressure. The more the ion is hydrated, the more heat is released. Degree of hydration depends on the size and charge of ion. The smaller the ion and the greater its charge, it will be the more hydrated.
ionic conductor → ionski vodič
Ionic conductor is a material that conducts electricity with ions as charge carriers.
ionophore → ionofore
Ionophore is a relatively small hydrophobic molecule that facilitates the transport of ions across lipid membranes. Most ionophores are produced by microorganisms. There are two types of ionophores: channel formers, which combine to form a channel in the membrane through which ions can flow; and mobile ion carriers, which transport ions across a membrane by forming a complex with the ion.
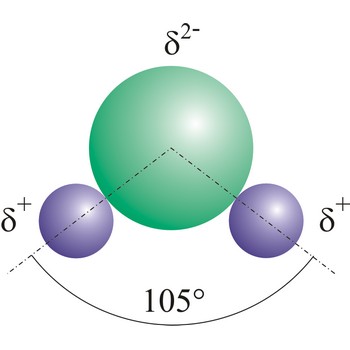
dipole molecule → dipolna molekula
Dipole molecules are created when mutual electronic pair at covalent bond is asymmetrical. If different atoms are bonded by a covalent bond, which can have different electron affinity, then the the atom with greater electron affinity will attract the electron pairs more strongly. In this way an asymmetrical distribution of negative charge appears in a molecule, so one part of the molecule becomes relatively negatively (the one closer to the electron pair) and the other becomes relatively positively charged.
dipole moment → dipolni moment
Electric dipole moment (μ) is a product of the positive charge and the distance between the charges. Dipole moments are often stated in debyes; The SI unit is the coulomb metre. In a diatomic molecule, such as HCl, the dipole moment is a measure of the polar nature of the bond; i.e. the extent to which the average electron charges are displaced towards one atom (in the case of HCl, the electrons are attracted towards the more electronegative chlorine atom). In a polyatomic molecule, the dipole moment is the vector sum of the dipole moments of the individual bonds. In a symmetrical molecule, such as tetrafluoromethane (CF4) there is no overall dipole moment, although the individual C-F bonds are polar.
dubnium → dubnij
Dubnium was discovered by workers at the Nuclear Institute at Dubna (USSR) and by workers at the University of California, Berkeley (USA) in 1967. The origin of the name dubnium is the Joint Nuclear Institute at Dubna, Russia, an institute heavily involved in the search for heavy elements. It is synthetic radioactive metal. Dubnium was made by bombarding californium-249 with a beam of nitrogen-15 ions. There are now five known isotopes of dubnium. The longest-lived is dubnium-262, with a half-life of 34 seconds.
Einstein equation → Einsteinova jednadžba
Einstein equation is the mass-energy relationship introduced by Albert Einstein in 1905 in the form E = mc2, where E is a quantity of energy, m its mass, and c is the speed of light. It presents the concept that energy possesses mass.
Citing this page:
Generalic, Eni. "Efektivni naboj jezgre." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table