saturated solution → zasićena otopina
Saturated solution is a solution that holds the maximum possible amount of dissolved material. When saturated, the rate of dissolving solid and that of recrystallisation solid are the same, and a condition of equilibrium is reached. The amount of material in solution varies with temperature; cold solutions can hold less dissolved solid material than hot solutions. Gases are more soluble in cold liquids than in hot liquids.
solubility product constant → konstanta produkta topljivosti
Solubility product constant (Ksp) (or the solubility product) is the product of the molar concentrations of the constituent ions, each raised to the power of its stoichiometric coefficient in the equilibrium equation. For instance, if a compound AaBb is in equilibrium with its solution
the solubility product is given by
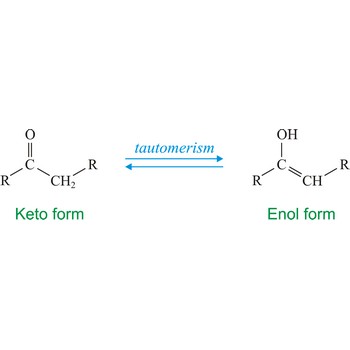
tautomerism → tautomerija
Tautomerism refers to equilibrium between two different structures of the same compound. Usually the tautomers differ in the point of attachment of a hydrogen atom. One of the most common examples of a tautomeric system is the equilibrium between a ketone (keto) and aldehyde (enol).
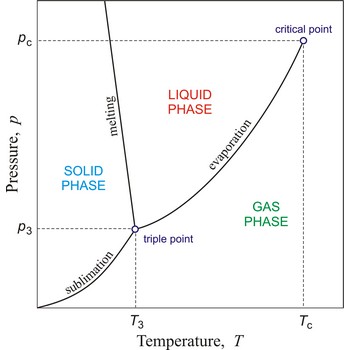
triple point → trojna točka
Triple point is the point in p,T space where the solid, liquid, and gas phases of a substance are in thermodynamic equilibrium.
unequal-arm balance → vaga s različitim krakovima
The lever principle on which these scales are constructed is based on the law of physics that at equilibrium the force applied at one end of the lever multiplied by the length of the arm (distance from the fulcrum to the point where the force is applied) must be equal to the product of the force acting at the opposite end of the lever and the length of the other arm.
The unequal-arm balance is preferred for work when large amounts are to be weighed.
van’t Hoff equation → van Hoffova jednadžba
Van’t Hoff equation is the equation expressing the temperature dependence on the equilibrium constant K of a chemical reaction:
where ΔrH° is the standard enthalpy of reaction, R the molar gas constant, and T the temperature.
water ion product → ionski produkt vode
Water ion product (Kw) is a concentration product of hydrogen and hydroxide ions. For the reaction:
the equilibrium expression would be:
Note that all pure liquid terms are omitted, hence H2O does not appear in the denominator. At 25 °C, Kw = 1.0×10-14 mol2dm-6 = (Ka)(Kb)
T-S diagram → T-S dijagram
The relationship between the temperature (T) and the salinity (S) of a seawater can be illustrated graphically on a T-S diagram, which is a simple, but powerful tool used in studies of seawater density, mixing, and circulation. In a T-S diagram, temperature is plotted along the vertical axis in degrees Celsius and salinity is measured along the horizontal axis in PSU (Practical Salinity Units). Seawater density is illustrated in the diagram by curved lines of constant density (isopycnals). Water tends to move horizontally throughout the deep ocean, moving along lines of equal density.
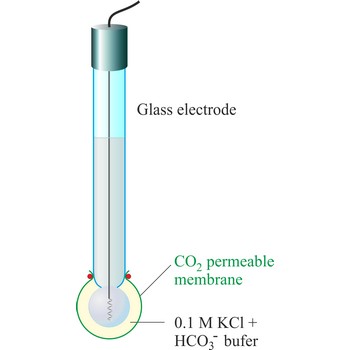
CO2 ion selective electrode → CO2 ion selektivna elektroda
The carbon dioxide ion selective electrode uses a gas-permeable membrane to separate the sample solution from the electrode internal solution. Dissolved carbon dioxide in the sample solution diffuses through the membrane until an equilibrium is reached between the partial pressure of CO2 in the sample solution and the CO2 in the internal filling solution. In any given sample the partial pressure of carbon dioxide will be proportional to the concentration of carbon dioxide. The diffusion across the membrane affects the level of hydrogen ions in the internal filling solution:
The hydrogen level of the internal filling solution is measured by the pH electrode located behind the membrane. The internal filling solution contains a high concentration of sodium bicarbonate (e.g. 0.1 mol/L NaHCO3) so that the bicarbonate level can be considered constant.
Citing this page:
Generalic, Eni. "Dinamička ravnoteža." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table