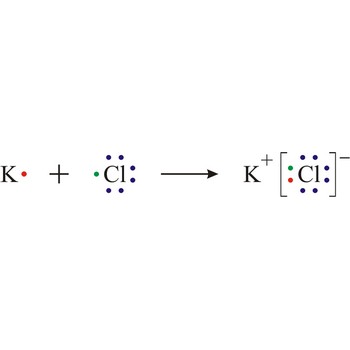chemical symbols → kemijski simboli
Chemical symbols are a derived way of showing elements in a formula or equation. Each symbol represents one atom and it usually consists of the first two letters of the Greek or Latin name of the element.
differential thermal analysis → diferencijalna termalna analiza
Differential thermal analysis (DTA) is a technique that is often used to analyze materials that react or decompose at higher temperatures. The difference in temperature between the sample and an inert reference material is monitored as both are heated in a furnace. Phase transitions and chemical reactions taking place in the sample on heating cause the temperature difference to become larger, at temperatures that are characteristic of the sample.
galvanic celll → galvanski članak
Galvanic cell (voltaic cell) is a simple device with which chemical energy is converted into electrical energy. Galvanic cells consist of two separate compartments called half cells containing electrolyte solutions and electrodes that can be connected in a circuit. Two dissimilar metals (e.g., copper and zinc) are immersed in an electrolyte. If the metals are connected by an external circuit, one metal is reduced (i.e., gains electrons) while the other metal is oxidized (i.e., loses electrons).
In the example above, copper is reduced and zinc is oxidized. The difference in the oxidation potentials of the two metals provides the electric power of the cell.
A voltaic cell can be diagrammed using some simple symbols. In the diagram the electrodes are on the outer side of the diagram and a vertical line (|) is used to separate the electrode from the electrolyte solution found in the compartment. A double vertical line (||) is used to separate the cell compartments and is symbolic of the salt bridge. Usually in a diagram the species oxidized is written to the left of the double slash. Here is an example of the Daniell cell:
The names refer to the 18th-century Italian scientists Alessandro Volta (1745-1827) and Luigi Galvani (1737-1798).
absolute error → apsolutna pogreška
Absolute error is a difference between the obtained value (O) and the real value (μ). It is shown in employed for measuring (g, cm3, ...). For example, if three replicate weights for an object are 1.00 g, 1.05 g, and 0.95 g, the absolute error can be expressed as ±0.05 g. Absolute error is also used to express inaccuracies; for example, if the "true value" is 1.11 g and the measured value is 1.00 g, the absolute error could be written as 1.00 g - 1.11 g = -0.11 g. Note that when absolute errors are associated with indeterminate errors, they are preceded by "±"; when they are associated with determinate errors, they are preceded by their sign.
activated complex → aktivirani kompleks
Activated complex is an intermediate structure formed in the conversion of reactants to products. The activated complex is the structure at the maximum energy point along the reaction path; the activation energy is the difference between the energies of the activated complex and the reactants.
allotropy → alotropija
Allotropy (Gr. allos, other, and tropos, manner) is the phenomenon of an element existing in two or more physical forms in the same physical state. The difference between the forms involves either crystaline structure (white, red and black phosphorus), the number of atoms in the molecule of a gas (diatomic oxygen and triatomic ozone), or the molecular structure of a liquid (liquid helium an helium II).
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C. This form allotropy is called enantiotropy. Form of allotropy, in which there is no transition temperature at which the two are in equilibrium, is called monotropy.
Allotropy does not apply to the substance existing in different physical states as, for example, when ice melts and changes from solid ice to liquid water.
Allotropy is generally restricted to describing polymorphic behaviour in elements, while polymorphism may refer to any material having multiple crystal structures.
amount concentration → količinska koncentracija
Amount concentration (also called molar concentration and in older literature molarity) is the amount of a given substance in a stated unit of a mixture, solution, or ore. The common unit is mole per cubic decimetre (moldm−3) or mole per litre (molL-1) sometimes denoted by M.
The concentration of an atom, ion, or molecule in a solution may be symbolised by the use of square brackets, as [Ca2+].
aqueous solution → vodena otopina
Aqueous solutions are those solutions where water is the solvent. An aqueous solution found in an equation describing a chemical reaction is denoted by the state symbol, (aq).
atomic number → atomski broj
Atomic number (Z) is a characteristic property of an element, equal to the number of protons in the nucleus. The atomic number and the element symbol are two alternate ways to label an element. In nuclide symbols, the atomic number is a leading subscript; for example, in 2He.
Citing this page:
Generalic, Eni. "Different o symbol." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table