conjugated protein → konjugirani protein
Conjugated proteins are proteins which have a prostetic group as a part of their structure which is bonded with one or more amino acids of the same protein.
crystal → kristal
Crystal is a solid with a regular geometric shape, having a characteristic internal structure and enclosed by symmetrically arranged plane surfaces, intersecting at definite and characteristic angles. In crystals the particles (atoms, ions, or molecules) have a regular three-dimensional repeating arrangement in space. This is called the crystal structure. The crystal lattice is the arrangement of points in space at which the particles are positioned.
beta-glucan → beta-glukan
Beta-glucans are are naturally occurring polysaccharides that contain only glucose as structural components, and are linked with β-glycosidic bonds. They is the most known powerful immune stimulant. The most active forms of β-glucans are those comprising D-glucose units with β(1→3) links and with short side-chains of D-glucose attached at the β(1→6) position. These are referred to as beta-1,3/1,6 glucan. They are a major component of soluble dietary fiber, which can be found in cereal grains (oats, barley, wheat), yeast, and certain mushrooms (shiitake, maitake).
borane → borani
Borane is any of the group of compounds of boron and hydrogen (B2H6, B4H10, B5H9, B5H11...), many of which can be prepared by action of acid on magnesium boride (Mg3B2). Boranes are a remarkable group of compounds in that their structures cannot be described using the conventional two-electron covalent bond model.
Bunsen burner → Bunsenov plamenik
Bunsen burner is a standard source of heat in the laboratory. German chemist Roberts Bunsen (1811-1899) improved the burner's design, which had been invented by Faraday, to aid his endeavors in spectroscopy. The Bunsen burner has a vertical metal tube through which a fine jet of fuel gas is directed. Air is drawn in through airholes near the base of the tube and the mixture is ignited and burns at the tube’s upper opening. The flow of this air is controlled by an adjustable collar on the side of the metal tube. When the whole is closed a yellow safety flame is displayed. Where as when the whole is open it displays a power dull blue flame with a faint blue outer flame with a vibrant blue core used u for combustion and hearting. The flame can reach temperatures of 1 500 °C.
crystallography → kristalografija
Crystallography is a science that studies structure, shapes, crystalline properties and laws of their creation.
D-lines → D-linije
D-lines are two close lines in the yellow region of the spectrum of sodium, having wavelengths 589.6 nm (D1) and 589.0 nm (D2). They were labeled as feature D in the solar spectrum by German optician Joseph von Fraunhofer (1787-1826). As they a prominent and easily recognized they are used as a standard in spectroscopy.
carbohydrate → ugljikohidrat
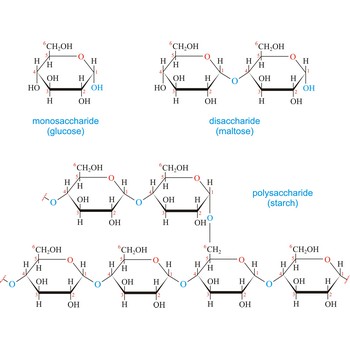
Carbohydrates (often called carbs for short) are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis. They are also known as saccharides, a term derived from the Latin word saccharum for sugar. Carbohydrates are the most abundant class of compounds in the biological world, making up more than 50 % of the dry weight of the Earth’s biomass. Every type of food we eat can have its energy traced back to a plant. Plants use carbon dioxide and water to make glucose, a simple sugar, in photosynthesis. Other carbohydrates such as cellulose and starch are made from the glucose. Light from the sun is absorbed by chlorophyll and this is converted to the energy necessary to biosynthesize carbohydrates
The term carbohydrate was applied originally to monosaccharides, in recognition of the fact that their empirical composition can be expressed as Cx(H2O)y. Later structural studies revealed that these compounds were not hydrates but the term carbohydrate persists.
Carbohydrates are generally classed as either simple or complex. Simple sugars, or monosaccharides, are carbohydrates that can’t be converted into smaller subunits by hydrolysis. Complex carbohydrates are made of two (disaccharides) or more (oligosaccharides, polysaccharides) simple sugars linked together by acetal (glycosidic) bonds and can be split into the former by hydrolysis.
carbon → ugljik
Carbon has been known since ancient times. The origin of the name comes from the Latin word carbo meaning charcoal. Graphite form of carbon is a black, odourless, slippery solid. Graphite sublimes at 3825 °C. Diamond form is a clear or colored; an extremely hard solid. C60 is Buckminsterfullerine. Carbon black burns readily with oxidants. Carbon is made by burning organic compounds with insufficient oxygen. There are close to ten million known carbon compounds, many thousands of which are vital to organic and life processes. Radiocarbon dating uses the carbon-14 isotope to date old objects.
derivative → derivat
Derivative is a compound that is derived from some other compound and usually maintains its general structure, e.g. trichlormethane (chloroform) is a derivative of methane.
Citing this page:
Generalic, Eni. "Cubic close-packed structure." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table