glutamic acid → glutaminska kiselina
Glutamic acid is an electrically charged amino acids. It is one of the two amino acids that contain a carboxylic acid group in its side chains. These acids play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins. Glutamic acid is commonly referred to as glutamate, because its carboxylic acid side chain will be deprotonated and thus negatively charged in its anionic form at physiological pH. Glutamic acid is referred to as a non-essential amino acid because a healthy human can synthesize all the glutamic acid needed for normal body function from other amino acids.
- Abbreviations: Glu, E
- IUPAC name: 2-aminopentanedioic acid
- Molecular formula: C5H9NO4
- Molecular weight: 147.13 g/mol
glass electrode → staklena elektroda
Glass electrode is a hydrogen-ion responsive electrode usually consisting of a bulb, or other suitable form, of special glass attached to a stem of high resistance glass complete with internal reference electrode and internal filling solution system. Glass electrode is also available for the measurement of sodium ions.
The glass electrode, which consists of a thin wall glass bulb, has an extremely high electrical resistance. The membrane of a typical glass electrode (with a thickness of 0.03 mm to 0.1 mm) has an electrical resistance of 30 MΩ to 600 MΩ. The surface of a glass membrane must be hydrated before it will function as a pH electrode. When a glass surface is immersed in an aqueous solution then a thin solvated layer (gel layer) is formed on the glass surface in which the glass structure is softer. This applies to both the outside and inside of the glass membrane.
The simplest explanation for the working of the thin glass electrode is that the glass acts as a weak acid (Glass-H).
The hydrogen ion activity of the internal solution is held constant. When a solution of different pH from the inside comes in contact with the outside of the glass membrane, the glass is either deprotonated or protonated relative to the inside of the glass. The difference in pH between solutions inside and outside the thin glass membrane creates electromotive force in proportion to this difference in pH.
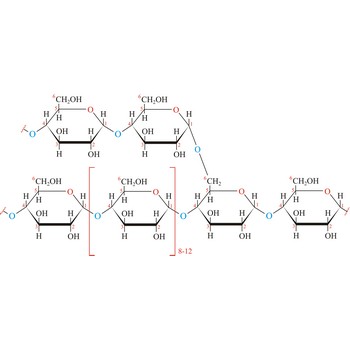
glycogen → glikogen
Glycogen (animal starch) is a polysaccharide that serves the same energy storage function in animals that starch serves in plants. Dietary carbohydrates not needed for immediate energy are converted by the body to glycogen for long term storage (principally in muscle and liver cells). Like amylopectin found in starch, glycogen is a polymer of α(1→4)-linked subunits of glucose, with α(1→6)-linked branches. Glycogen molecules are larger than those of amylopectin (up to 100 000 glucose units) and contain even more branches. Branch points occur about every 10 residues in glycogen and about every 25 residues in amylopectin. The branching also creates lots of ends for enzyme attack and provides for rapid release of glucose when it is needed.
hexagonal close-packed structure → heksagonska gusta slagalina
In a hexagonal close-packed (hcp) arrangement of atoms, the unit cell consists of three layers of atoms. The top and bottom layers (a) contain six atoms at the corners of a hexagon and one atom at the center of each hexagon. The middle layer (b) contains three atoms nestled between the atoms of the top and bottom layers, hence, the name close-packed. The hexagonal close packed structure can be made by piling layers in the a-b-a-b-a-b... sequence.
hexagonal lattice → heksagonska rešetka
Hexagonal lattice has lattice points at the twelve corners of the hexagonal prism and at the centers of the two hexagonal faces of the unit cell. It has unit cell vectors a=b≠c and interaxial angles α=β=90° and γ=120°.
hybrid orbital → hibridne orbitale
Hybrid orbital is an orbital created by mixing together atomic orbitals to form an equal number of new hybrid atomic orbitals. For example, a common hybridization is sp3 where s orbital combine with a three p orbitals to form four new orbitals. After hybridization, all hybrid orbitals have the same energy, lower than p orbitals, but higher than s orbitals.
hydrosphere → hidrosfera
Hydrosphere (from the Greek for water sphere) is a discontinuous layer of water on, under, and over the Earth's surface. It includes all liquid and frozen surface waters, groundwater held in soil and rock, and atmospheric water vapour. Water continuously circulates between these reservoirs in what is called the hydrologic cycle, which is driven by energy from the Sun.
| Reservoir | V / 106 km3 | w / % |
|---|---|---|
| oceans | 1 370.0 | 97.25 |
| ice caps and glaciers | 29.0 | 2.05 |
| groundwater | 9.5 | 0.68 |
| lakes, rivers | 0.127 | 0.01 |
| soil moisture | 0.065 | 0.005 |
| atmosphere (as liquid equivalent of water vapour) | 0.013 | 0.001 |
| biosphere | 0.0006 | 0.00004 |
| TOTAL | 1 408.7 | 100 |
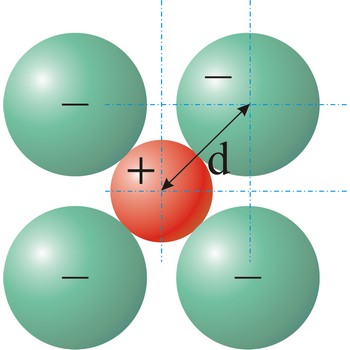
ionic radius → ionski radijus
Ionic radius is the radius of anions and cations in crystalline ionic compounds, as determined by consistently partitioning the center-to-center distance of ions in those compounds. In general, negative ions have larger ionic radii than positive ions.
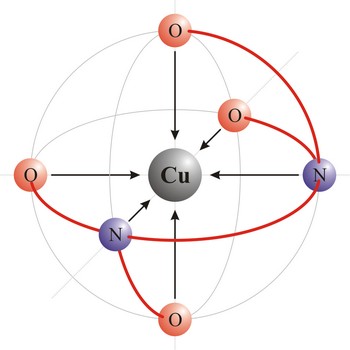
ligand → ligand
Ligand is an ion (F-, Cl-, Br-, I-, S2-, CN-, NCS-, OH-, NH2-) or molecule (NH3, H2O, NO, CO) that donates a pair of electrons to a metal atom or ion in forming a coordination complex. The main way of classifying ligands is by the number of points at which they are attached to, or bound to, the metal center. This is the denticity. Ligands with one potential donor atom are monodentate. Polydentate ligand is a ligand that is attached to a central metal ion by bonds from two or more donor atoms. Ligands with more than one potential donor atom are known as ambidentate, such as the thiocyanate ion, NCS-, which can bind to the metal center with either the nitrogen or sulphur atoms. Chelating ligands are those polydentate ligands which can form a ring including the metal atom.
lime → živo vapno
Lime (or quicklime) is the common name for calcium oxide (CaO). It is manufactured from limestone, CaCO3, by heating it to a high temperature (about 1 000 °C). At this temperature carbon dioxide, CO2, is released from the limestone creating calcium oxide, CaO.
A further process involves adding water in a process known as hydrating, which produces hydrated, or slaked lime [Ca(OH)2].
Citing this page:
Generalic, Eni. "Create polyline from center cicle." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table