thixotropic fluid → tiksotropni fluid
Thixotropic fluid (thixotropy) is a liquid that becomes less viscous when stirred. Paint and printing inks are thixotropic fluids, they are formulated so that they flow more freely when brushed or rolled.
Bunsen, Robert Wilhem → Bunsen, Robert Wilhem
Robert Wilhem Bunsen (1811-1899) is a German chemist who held professorships at Kassel, Marburg and Heidelberg. His early researches on organometallic compound of arsenic cost him an eye in an explosion. Bunsen's most important work was in developing several techniques used in separating, identifying, and measuring various chemical substances. He also improvement chemical battery for use in isolating quantities of pure metals - Bunsen battery.
The essential piece of laboratory equipment that has immortalized the name of Bunsen was not invented by him. Bunsen improved the burner's design, which had been invented by Faraday, to aid his endeavors in spectroscopy. Use of the Bunsen burner in conjunction with a glass prism led to the development of the spectroscope in collaboration with the German physicist Gustav Kirchoff and to the spectroscopic discovery of the elements rubidium (1860) and cesium (1861).
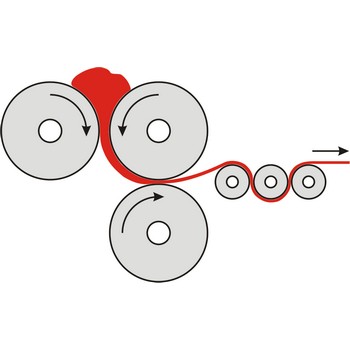
calendering → kalandriranje
Calendering is the process of forming materials to make a film/sheet by passing them through a series of hot rollers.
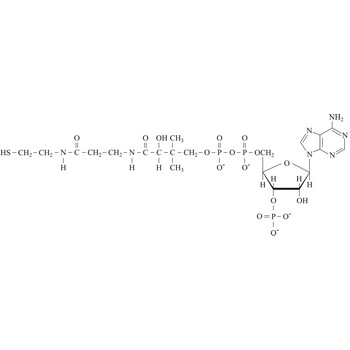
coenzyme a → koenzim a
Coenzyme A (CoA) is an essential metabolic cofactor synthesized from cysteine, pantothenate (vitamin B5), and ATP. CoA plays important roles in many metabolic pathways, including the tricarboxylic acid (TCA) cycle, and the synthesis and oxidation of fatty acids. One of the main functions of CoA is the carrying and transfer of acyl groups. Acylated derivatives (acetyl-CoA) are critical intermediates in many metabolic reactions.
glutamic acid → glutaminska kiselina
Glutamic acid is an electrically charged amino acids. It is one of the two amino acids that contain a carboxylic acid group in its side chains. These acids play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins. Glutamic acid is commonly referred to as glutamate, because its carboxylic acid side chain will be deprotonated and thus negatively charged in its anionic form at physiological pH. Glutamic acid is referred to as a non-essential amino acid because a healthy human can synthesize all the glutamic acid needed for normal body function from other amino acids.
- Abbreviations: Glu, E
- IUPAC name: 2-aminopentanedioic acid
- Molecular formula: C5H9NO4
- Molecular weight: 147.13 g/mol
hafnium → hafnij
Hafnium was discovered by Dirk Coster (Denmark) and Georg Karl von Hevesy (Hungary) in 1923. The origin of the name comes from the Latin name Hafnia meaning Copenhagen. It is silvery, ductile metal. Exposed surfaces form oxide film. Resists alkalis and acids (except HF). Toxic. Metal ignites and burns readily. Hafnium is obtained from mineral zircon or baddeleyite. Used in reactor control rods because of its ability to absorb neutrons.
high fructose corn syrup → visoko fruktozni kukuruzni sirup
High fructose corn syrup (HFCS) is commonly used in place of sugar in foods and drinks. Corn starch is hydrolyzed to glucose, which is then treated with glucose isomerase to produce a fructose-rich mixture. HFCS is available in a number of forms, named according to the percentage of fructose they contain, HFCS-55 for instance contains 55 % fructose and 45 % glucose. The enhanced sweetness, low cost and ease of use are the main reasons why manufacturers now prefer to use high fructose corn syrup instead of sugar.
Gratzel solar cell → Gratzelova sunčeva ćelija
Grätzel solar cell is photoelectrochemical cell, developed by Michael Grätzel and collaborators, simulates some characteristics of the natural solar cell, which enables photosynthesis take place. In natural solar cell the chlorophyll molecules absorb light (most strongly in the red and blue parts of the spectrum, leaving the green light to be reflected). The absorbed energy is sufficient to knock an electron from the excited chlorophyll. In the further transport of electron, other molecules are involved, which take the electron away from chlorophyll. In Grätzel cell, the tasks of charge-carrier generation and transport are also assigned to different species.
His device consists of an array of nanometre-sized crystallites of the semiconductor titanium dioxide, welded together and coated with light-sensitive molecules that can transfer electrons to the semiconductor particles when they absorb photons. So, light-sensitive molecules play a role equivalent to chlorophyll in photosynthesis. In Grätzel cell, the light-sensitive molecule is a ruthenium ion bound to organic bipyridine molecules, which absorb light strongly in the visible range; titanium dioxide nanocrystals carry the received photoexcited electrons away from electron donors. On the other hand, a donor molecule must get back an electron, so that it can absorb another photon. So, this assembly is immersed in a liquid electrolyte containing molecular species (dissolved iodine molecules) that can pick up an electron from an electrode immersed in the solution and ferry it to the donor molecule. These cells can convert sunlight with efficiency of 10 % in direct sunlight and they are even more efficient in diffuse daylight.
inert electrode → inertna elektroda
Inert electrode is an electrode that serves only as a source or sink for electrons without playing a chemical role in the electrode reaction. Precious metals, mercury, and carbon are typically used as inert electrodes. The inert nature of the electrode can sometimes be questioned. While the electrode may not take part in the reaction as a reactant or product, it still can act as an electrocatalyst.
Citing this page:
Generalic, Eni. "Cost per roll." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table