cyclic voltammetry → ciklička voltametrija
Cyclic voltammetry (CV) is an electrochemical measuring technique used for the determination of the kinetics and mechanism of electrode reactions. The potential of the working electrode is controlled (typically with a potentiostat) and the current flowing through the electrode is measured. It is a linear-weep voltammetry with the scan continued in the reverse direction at the end of the first scan. This cycle can be repeated a number of times, and is used for corrosion studies.
voltametry → voltametrija
Voltametry is a common name for a large group of instrumental techniques which are based on measuring the electric current formed by a continuous potential shifting on the electrodes.
cyclic compound → ciklički spoj
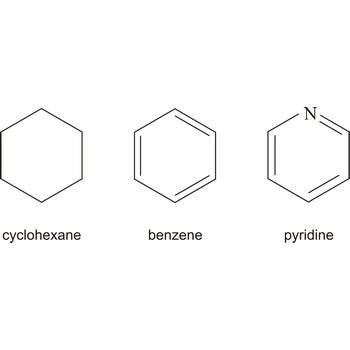
Cyclic describing a compound that has a ring of atoms in its molecules. In homocyclic compounds all the atoms in the ring are of the same type, e.g. benzene (C6H6) and cyclohexane (C6H12). These two examples are also examples of carbocyclic compounds; i.e. the rings are made of carbon atoms. If different atoms occur in the ring, as in pyridine (C5H5N), the compound is said to be heterocyclic.
alicyclic → aliciklički spojevi
Alicyclic compounds are aliphatic compounds with a ring of atoms. They have CnH2n general formula (e.g. cyclohexane C6H12).
aliphatic compound → alifatski spoj
Aliphatic compounds are acyclic or cyclic, saturated or unsaturated carbon compounds, excluding aromatic compounds.
anomer → anomer
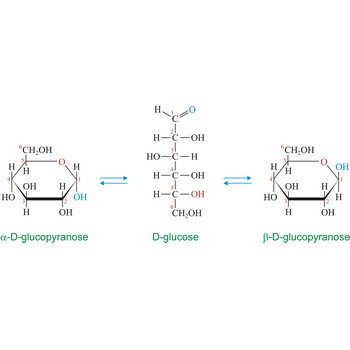
Anomers are diastereoisomers of cyclic forms of sugars or similar molecules differing in the configuration at the anomeric carbon (C-1 atom of an aldose or the C-2 atom of a 2-ketose). The cyclic forms of carbohydrates can exist in two forms, α- and β- based on the position of the substituent at the anomeric center. Anomer are designated α if the configuration at the anomeric carbon is the same as that at the reference asymmetric carbon in a Fischer projection. If the configuration differs the anomer is designated β. For example, α-D-glucopyranose and β-D-glucopyranose, the two cyclic forms of glucose, are anomers.
aromatic compounds → aromatski ugljikovodici
Aromatic compounds are a major group of unsaturated cyclic hydrocarbons containing one or more rings, typified by benzene, which has a 6-carbon ring containing three double bonds. All the bonds in benzene (C6H6) are the same length intermediate between double and single C-C bonds. The properties arise because the electrons in the p-orbitals are delocalised over the ring, giving extra stabilization energy of 150 kJ/mol over the energy of Kekulé structure. Aromatic compounds are unsaturated compounds, yet they do not easily partake in addition reactions.
Historical use of the term implies a ring containing only carbon (e.g., benzene, naphthalene), but it is often generalized to include heterocyclic structures such as pyridine and thiophene.
cyclization → ciklizacija
Cyclization is the formation of a cyclic compound from an open-chain compound.
cycloalkanes → cikloalkani
Cycloalkanes are cyclic saturated hydrocarbons containing a ring of carbon atoms joined by single bonds. They have the general formula CnH2n, for example cyclohexane, C6H12. In general, they behave like the alkanes but are rather less reactive.
heterocyclic compounds → heterociklički spojevi
Heterocyclic compounds are cyclic compounds having as ring members atoms of at least two different elements, e.g., quinoline, 1,2-thiazole.
Citing this page:
Generalic, Eni. "Ciklička voltametrija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table