bomb calorimeter → kalorimetrijska bomba
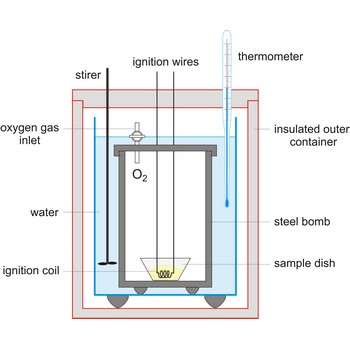
Bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of samples which can be burned in oxygen. Four essential parts are required in any bomb calorimeter:
- a bomb or vessel in which the combustible charges can be burned,
- a bucket or container for holding the bomb in a measured quantity of water, together with a stirring mechanism,
- an insulating jacket to protect the bucket from transient thermal stresses during the combustion process, and
- a thermometer or other sensor for measuring temperature changes within the bucket.
eutectic → eutektik
Eutectic is a solid solution consisting of two or more substances and having the lowest freezing point of any possible mixture of these components.
Eutectic point is the lowest temperature at which the eutectic mixture can exist in a liquid phase. A liquid having the eutectic composition will freeze at a single temperature without a change of composition.
evaporation → isparavanje
Evaporation is the change of state of a liquid into a vapour at a temperature below the boiling point of the liquid.
Fermi level → Fermijev nivo
Fermi level is the highest energy of occupied states in a solid at zero temperature. The Fermi level in conductors lies in the conduction band, in insulators it lies in the valence band, and in semiconductors it falls in the gap between the conduction band and the valence band. It was named after the Italian physicst Enrico Fermi (1901 - 1954).
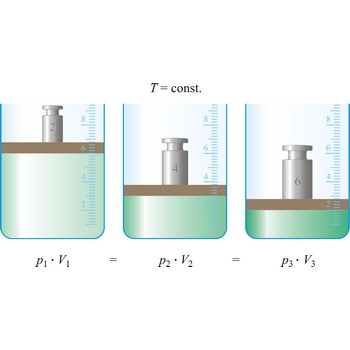
Boyle’s law → Boyleov zakon
Boyle’s law (sometimes referred to as the Boyle-Mariott’s law) is the empirical law, exact only for an ideal gas, which states that the volume of a gas is inversely proportional to its pressure at constant temperature.
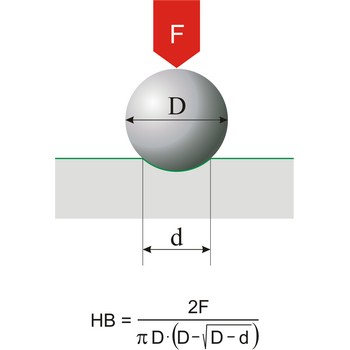
Brinell hardness → Brinellova tvrdoća
Brinell hardness is a scale for measuring the hardness of metals introduced around 1900 by Swedish metallurgist Johan Brinell (1849-1925). A small chromium steel ball is pressed into the surface of the metal by a load of known weight. The loading force is in the range of 300 N to 30 000 N. The ratio of the mass of the load in kilograms to the area of the depression formed in square millimetres is the Brinell Hardness Number.
Bunsen burner → Bunsenov plamenik
Bunsen burner is a standard source of heat in the laboratory. German chemist Roberts Bunsen (1811-1899) improved the burner's design, which had been invented by Faraday, to aid his endeavors in spectroscopy. The Bunsen burner has a vertical metal tube through which a fine jet of fuel gas is directed. Air is drawn in through airholes near the base of the tube and the mixture is ignited and burns at the tube’s upper opening. The flow of this air is controlled by an adjustable collar on the side of the metal tube. When the whole is closed a yellow safety flame is displayed. Where as when the whole is open it displays a power dull blue flame with a faint blue outer flame with a vibrant blue core used u for combustion and hearting. The flame can reach temperatures of 1 500 °C.
fire-damp → plin praskavac
Fire-damp is a mixture of two volume parts of hydrogen and one volume part of oxygen which, if set on fire, strongly explodes, the flame giving of a very high temperature (2 000 °C).
flash point → temperatura zapaljenja
Flash point is the lowest temperature at which a liquid or volatile solid gives off vapour sufficient to form an ignitable mixture with the air near the surface of the liquid or within the test vessel (NFPA).
fluid mechanics → mehanika fluida
Fluid mechanics is the study of various properties of the fluid (liquids and gases): velocity, pressure, density and temperature, as functions of space and time.
Citing this page:
Generalic, Eni. "Celsius temperature scale." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table