allotropy → alotropija
Allotropy (Gr. allos, other, and tropos, manner) is the phenomenon of an element existing in two or more physical forms in the same physical state. The difference between the forms involves either crystaline structure (white, red and black phosphorus), the number of atoms in the molecule of a gas (diatomic oxygen and triatomic ozone), or the molecular structure of a liquid (liquid helium an helium II).
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C. This form allotropy is called enantiotropy. Form of allotropy, in which there is no transition temperature at which the two are in equilibrium, is called monotropy.
Allotropy does not apply to the substance existing in different physical states as, for example, when ice melts and changes from solid ice to liquid water.
Allotropy is generally restricted to describing polymorphic behaviour in elements, while polymorphism may refer to any material having multiple crystal structures.
antifreeze → antifriz
Antifreeze is a substance added to the liquid (usually water) in the cooling systems of internal-combustion engines to lower its freezing point so that it does not solidify at sub-zero temperatures. The commonest antifreeze is ethane- 1.2-diol (ethylene glycol).
Arrhenius equation → Arheniusova jednadžba
In 1889, Svante Arrhenius explained the variation of rate constants with temperature for several elementary reactions using the relationship
where the rate constant k is the total frequency of collisions between reaction molecules A times the fraction of collisions exp(-Ea/RT) that have an energy that exceeds a threshold activation energy Ea at a temperature of T (in kelvin). R is the universal gas constant.
Avogadro’s law → Avogadrov zakon
Avogadro’s law: Equal volumes of all gases contain equal numbers of molecules at the same pressure and temperature. The law, often called Avogadro’s hypothesis, is true only for ideal gases. It was proposed in 1811 by Italian chemist Amadeo Avogadro (1776-1856).
boiling point → vrelište
Boiling point is the temperature at which the vapour pressure of a liquid is equal to the external pressure on the liquid. The standard boiling point is the temperature at which the vapour pressure of a liquid equals standard pressure (101 325 Pa).
Boudouard’s equilibrium → Boudouardova ravnoteža
Boudouard’s equilibrium is established when carbon dioxide reacts with carbon. Because of reactions endothermity the temperature increase shifts the reaction rightwards and the temperature reduction leftwards.
catalytic hydrogenation → katalitičko hidrogeniranje
Catalytic hydrogenation is the infusing of unsaturated or impure hydrocarbons with hydrogen gas at controlled temperatures and pressures and in the presence of a catalyst for the purpose of obtaining saturated hydrocarbons and/or removing various impurities such as sulphur and nitrogen.
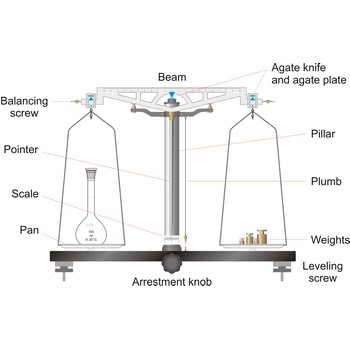
balance → vaga
Balance is an instrument to measure the mass (or weight) of a body. Balance beam type scales are the oldest type and measure weight using a fulcrum or pivot and a lever with the unknown weight placed on one end of the lever, and a counterweight applied to the other end. When the lever is balanced, the unknown weight and the counterweight are equal. The equal-arm balance consists of two identical pans hung from either end of a centrally suspended beam. The unequal-arm balance is made with one arm of the balance much longer than the other.
More modern substitution balances use the substitution principle. In this calibrated weights are removed from the single lever arm to bring the single pan suspended from it into equilibrium with a fixed counter weight. The substitution balance is more accurate than the two-pan device and enables weighing to be carried out more rapidly.
Electromagnetic force restoration balances also use a lever system but a magnetic field is used to generate the force on the opposite end of the lever and balance out the unknown mass. The current used to drive the magnetic coil is proportional to the mass of the object placed on the platform.
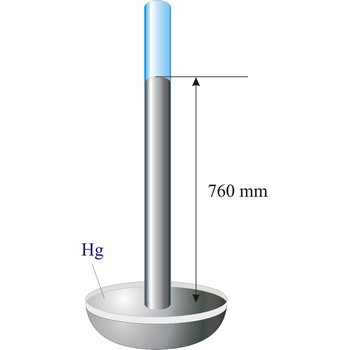
barometer → barometar
Barometer is an instrument that measures atmospheric pressure. A mercury barometer is a closed tube filled with mercury inverted in a mercury reservoir. The height of the mercury column indicates atmospheric pressure (with 1 atm = 760 mm of mercury). An aneroid barometer consists of an evacuated container with a flexible wall. When atmospheric pressure changes, the wall flexes and moves a pointer which indicates the changing pressure on a scale.
coagulation → koaguliranje
Coagulation is a process of colloid particles merging into bigger ones. By removing the charge form a colloid ion, by increasing the temperature or by increasing electrolyte concentration, colloid particles will gather into bigger groups precipitate will emerge. Precipitate that is formed in this way (coagulate) is amorphic and considerably polluted with adsorbed pollutants.
Citing this page:
Generalic, Eni. "Celsius temperature scale." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table