saturated fat → zasićena mast
Saturated fats are fats in foods that are solid at room temperature. They come chiefly from animal sources (beef, whole-milk dairy products, dark meat poultry) but also from tropical vegetable oils (coconut, palm).
saturated solution → zasićena otopina
Saturated solution is a solution that holds the maximum possible amount of dissolved material. When saturated, the rate of dissolving solid and that of recrystallisation solid are the same, and a condition of equilibrium is reached. The amount of material in solution varies with temperature; cold solutions can hold less dissolved solid material than hot solutions. Gases are more soluble in cold liquids than in hot liquids.
Schellbach’s burette → Schellbachova bireta
When colourless liquids are used, parallax mistake is avoided by use of Schellbach’s burette. On the inside wall opposite to graduation scale it has a melted in ribbon from milky glass in the middle of which a blue line is found. The level of liquid is now spotted very easily because of light breaking in the meniscus blue line now looks like a double spike.
semiconductor → poluvodič
Semiconductor is a material in which the highest occupied energy band (valence band) is completely filled with electrons at T = 0 K, and the energy gap to the next highest band (conduction band) ranges from 0 to 4 or 5 eV. With increasing temperature electrons are excited into the conduction band, leading to an increase in the electrical conductivity.
silicon → silicij
Silicon was discovered by Jöns Jacob Berzelius (Sweden) in 1824. The origin of the name comes from the Latin word silicis meaning flint. Amorphous form of silicon is brown powder; crystalline form has grey metallic appearance. Solid form unreactive with oxygen, water and most acids. Dissolves in hot alkali. Silica dust is a moderately toxic acute irritant. Silicon makes up major portion of clay, granite, quartz and sand. Commercial production depends on a reaction between sand (SiO2) and carbon at a temperature of around 2200 °C. Used in glass as silicon dioxide (SiO2). Silicon carbide (SiC) is one of the hardest substances known and used in polishing. Also the crystalline form is used in semiconductors.
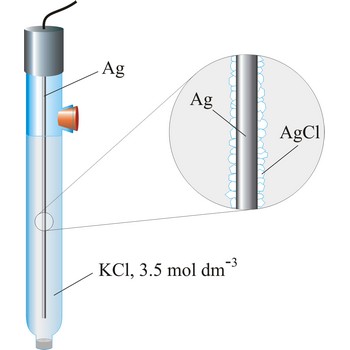
silver/silver-chloride electrode → srebro/srebrov klorid elektroda
Silver/silver-chloride electrode is by far the most common reference type used today because it is simple, inexpensive, very stable and non-toxic. It is mainly used with saturated potassium chloride electrolyte, but can be used with lower concentrations such as 3.5 mol dm-3 or 1 mol dm-3 potassium chloride. Silver/silver-chloride electrode is a referent electrode based on the following halfreaction
| Potential vs. SHE / V | ||
|---|---|---|
| t / °C | 3.5 mol dm-3 | sat. solution |
| 15 | 0.212 | 0.209 |
| 20 | 0.208 | 0.204 |
| 25 | 0.205 | 0.199 |
| 30 | 0.201 | 0.194 |
| 35 | 0.197 | 0.189 |
solubility → topljivost
Solubility is the maximum amount of solute that dissolves in a given quantity of solvent at a specific temperature. Generally, for a solid in a liquid, solubility increases with temperature; for a gas, solubility decreases. Common measures of solubility include the mass of solute per unit mass of solution (mass fraction), mole fraction of solute, molality, molarity, and others.
stratosphere → stratosfera
Stratosphere is the part of the earth’s atmosphere extending from the top of the troposphere (typically 10 km to 15 km above the surface) to about 50 km. It is characterised by an increase in temperature with increasing altitude.
supercritical fluid → superkritični fluid
Supercritical fluid is any substance above its critical temperature and critical pressure (see phase diagram). It shows unique properties that are different from those of either gases or liquids under standard conditions. A supercritical fluid has both the gaseous property of being able to penetrate anything, and the liquid property of being able to dissolve materials into their components. Solublity increases with increasing density (i.e. with increasing pressure). An example of this is naphthalene which is practically insoluble in low pressure carbon dioxide. At 100 bar the solubility is 10 g/L and at 200 bar it is 50 g/L. Rapid expansion of supercritical solutions leads to precipitation of a finely divided solid.
superfluid helium → superfluidni helij
Superfluidity in helium-4 was discovered in 1938 by the Soviet physicist Pyotr Leonidovich Kapitsa. Helium-4 exhibits superfluidity when it is cooled below 2.18 K (-270.97 C), which is called the lambda (λ) point. At these temperatures, helium-4 exhibits the characteristics of two distinct fluids, one of which appears to flow without friction. An extensive series of experiments showed that in this state of helium, called helium II (He II), there is an apparent enormous rise in heat conductivity, at an increase rate of about three million. Another unusual property of He II is its mobile, rapid flow through capillaries or over the rim of its containment vessel as a thin film that exhibits no measurable viscosity and appears unaffected by the forces of gravity or evaporation and condensation.
Citing this page:
Generalic, Eni. "Celsius temperature scale." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table