state of matter → agregatno stanje
State of matter is one of the tree physical states in which matter can exist, i.e. solid, liquid or gas. Plasma is sometimes regarded as the fourth state of matter. By means of heating a solid substance will cross to liquid state at its melting point. If we heat up a liquid and beyond, at its boiling point it will cross to gaseous state - vapour.
stereoisomer → stereoizomer
Stereoisomers are compounds that have identical chemical constitution, but differ as regards the arrangement of the atoms or groups in space. Stereoisomers fall into two broad classes: optical isomers (enantiomers) and geometric isomers (cis-trans).
stratosphere → stratosfera
Stratosphere is the part of the earth’s atmosphere extending from the top of the troposphere (typically 10 km to 15 km above the surface) to about 50 km. It is characterised by an increase in temperature with increasing altitude.
structural formula → strukturna formula
Structural formula is a two dimensional representations of the arrangement of the atoms in molecules. Atoms are represented by their element symbols and covalent bonds are represented by lines. The symbol for carbon is often not drawn.
styrene → stiren
Styrene is an unsaturated hydrocarbon (C6H5OC2H3O) colourless, toxic liquid with a strong aromatic aroma. It is soluble in alcohol, ether, acetone, and carbon disulfide, but dissolves only slightly in water. It is used to make plastics such as polystyrene, ABS, styrene-butadiene rubber styrene-butadiene latex and unsaturated polyesters.
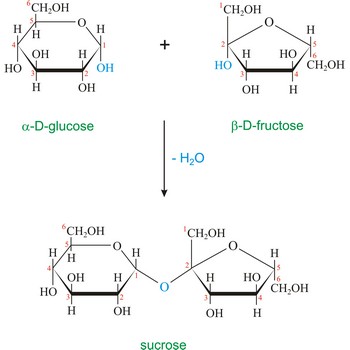
sucrose → saharoza
Sucrose (saccharose), or ordinary table sugar, is a disaccharide in which α-D-glucopyranose and β-D-fructofuranose are joined at their anomeric carbons by a glycosidic bond. There are no hemiacetals remaining in the sucrose and therefore sucrose is not a reducing sugar and does not exhibit mutarotation. Sugar is a white crystalline sweet compound found in many plants and extracted from sugar cane and sugar beet. It is used as a sweetening agent in food and drinks. If heated to 200 °C, sucrose becomes caramel. When sucrose is hydrolyzed it forms an equimolar mixture of glucose and fructose. This mixture of monosaccharides is called invert sugar. Honeybees have enzymes called invertases that catalyze the hydrolysis of sucrose. Honey, in fact, is primarily a mixture of glucose, fructose, and sucrose.
sugar → šećer
Sugar is any of a group of water-soluble carbohydrates of relatively low molecular weight and typically having a sweet taste. The group comprises mainly monosaccharides (glucose, fructose, galactose), disaccharides (sucrose, lactose, maltose), and trisaccharides (raffinose). Many monosaccharides and disaccharides fairly commonly found in nature bear names reflecting the source from which they were first isolated. For example, glucose is also known as grape sugar, lactose as milk sugar, and maltose as malt sugar. In everyday usage, the name is often used to refer specifically to sucrose (table sugar, cane sugar, beet sugar).
supercritical fluid → superkritični fluid
Supercritical fluid is any substance above its critical temperature and critical pressure (see phase diagram). It shows unique properties that are different from those of either gases or liquids under standard conditions. A supercritical fluid has both the gaseous property of being able to penetrate anything, and the liquid property of being able to dissolve materials into their components. Solublity increases with increasing density (i.e. with increasing pressure). An example of this is naphthalene which is practically insoluble in low pressure carbon dioxide. At 100 bar the solubility is 10 g/L and at 200 bar it is 50 g/L. Rapid expansion of supercritical solutions leads to precipitation of a finely divided solid.
T-shaped molecular geometry → T-oblik geometrije molekule
T-shape is a molecular geometry that results when there are 3 bonds and 2 lone pairs around the central atom in the molecule. The atoms bonded to the central atom lie at the ends of a T with 90° angles between them. Molecules with an trigonal bipyramidal electron pair geometries have sp3d (or dsp3) hybridization at the central atom. ICl3 has a T-shaped molecular geometry.
Citing this page:
Generalic, Eni. "Blast furnace glossary." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table