proton → proton
Proton is a stable elementary particle of unit positive charge and spin 1/2. Protons and neutrons, which are collectively called nucleons, are the constituents of the nucleus.
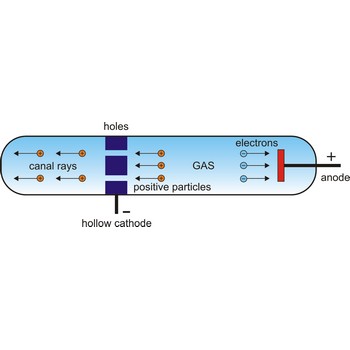
In 1886, German physicist Eugene Goldstein (1850-1930) discovered positive particles by using a modified Crookes tube with holes in the cathode in an evacuated tube. When cathode rays were given off in one direction toward the anode, other rays found their way through the holes in the cathode and sped off in the opposite direction. Since these other rays traveled in the direction opposite to the negatively charged cathode rays, it seemed that they must be composed of positively charged particles. Rutherford suggested that this fundamental positive particle be called the proton.
relative molecular mass → relativna molekularna masa
Relative molecular mass (Mr) is the ratio of the average mass per molecule or specified entity of a substance to 1/12 of the mass of nuclide 12C. Also called molecular weight. It is equal to the sum of the relative atomic masses of all the atoms that comprise a molecule. For example
Mr(H2SO4) = 2·Ar(H) + Ar(S) + 4·Ar(O)
= 2·1.0079 + 32.066 + 4·15.999
= 2.0158 + 32.066 + 63.996
= 98.078
spectrophotometer → spektrofotometar
Spectrophotometer is an instrument for measuring the amount of light absorbed by a sample.
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
thallium → talij
Thallium was discovered by Sir William Crookes (England) in 1861. The origin of the name comes from the Greek word thallos meaning green twig or green shoot. It is soft grey metal that looks like lead. Tarnishes in moist air. Reacts in heated moist air and in acids. Compounds highly toxic by inhalation or ingestion. Cumulative effects. Thallium is found in iron pyrites. Also in crookesite, hutchinsonite and lorandite. Most is recovered from the by-products of lead and zinc refining. Its compounds are used in rat and ant poisons. Also for detecting infrared radiation.
ununbium → ununbij
Ununbium was discovered by S. Hofmann et al. collaboration at the Heavy Ion Research Laboratory (Gesellschaft für Schwerionenforschung, GSI) in Darmstadt, Germany in February 1996. The new element has not yet been officially named, but it is known as ununbium, according to the system designated by the IUPAC for naming new elements. It is synthetic radioactive metal. Using the electromagnetic velocity filter SHIP, fusion-like residues of the reaction of 70Zn with enriched 208Pb targets were measured. Two chains of localized alpha-emitters were identified as originating with 277112 + 1n.
ununquadium → ununkvadij
The discovery of ununquadium was reported informally in January 1999 following experiments towards the end of December 1998 involving scientists at Dubna (Joint Institute for Nuclear Research) in Russia and the Lawrence Livermore National Laboratory, USA. The new element has not yet been officially named, but it is known as ununquadium, according to the system designated by the IUPAC for naming new elements. It is synthetic radioactive metal. Only few atoms of element 114 (289114) has ever been made (through a nuclear reaction involving fusing a calcium atom with a plutonium atom) isolation of an observable quantity has never been achieved.
unununium → unununij
Unununium was discovered by S. Hofmann et al. collaboration at the Heavy Ion Research Laboratory (Gesellschaft für Schwerionenforschung, GSI) in Darmstadt, Germany in December 1994. The new element has not yet been officially named, but it is known as unununium, according to the system designated by the IUPAC for naming new elements. It is synthetic radioactive metal. In bombardments of 209Bi targets with 64Ni using the velocity selector SHIP facility to discriminate in favor of the fused product, 272111 + 1n, three sets of localized alpha-decay chains were observed with position-sensitive detectors.
wavenumber → valni broj
Wavenumber is the number of wave cycles per unit distance.
There are unfortunately two different definitions of the wavenumber.
Wavenumber, k, is most frequently defined as
with wavelength λ, phase velocity of wave vp, and angular frequency ω.
Less frequently it is defined simply as
One must be careful to note which definition is in use. Wavenumbers are used extensively in infrared spectroscopy, and usually have units of cm-1.
work → rad
Work is the energy required to move an object against an opposing force. Work is usually expressed as a force times a displacement.
When a constant force F acts on a point-like object while the object moves through a displacement s, the force does work W on the object. If force and displacement are at a constant angle Θ to each other, the work is expressed by the scalar product of these two vectors:
When the force F on a point-like object is not constant that is, it depends on the position of the object, the work done by force while object moves from initial position with coordinates (xi, yi, zi) to final position with coordinates (xf, yf, zf)is given by expression:
Where Fx, Fy and Fz are scalar components of the force.
SI unit for work is joule (J); 1 J = 1 Nm = 1 kg m2 s-2. The electron-volt (eV) is commonly used in atomic and nuclear physics.
Citing this page:
Generalic, Eni. "Atomska apsorpcijska spektroskopija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table