einsteinium → einsteinij
Einsteinium was discovered by Albert Ghiorso (USA) in 1952. Named in honour of Albert Einstein (1879-1955). It is synthetic radioactive metal. Einsteinium was made by bombarding uranium with neutrons.
Beer’s law → Beerov zakon
Beer’s law (or Beer-Lambert law) is the functional relationship between the quantity measured in an absorption method (A) and the quantity sought, the analyte concentration (c). As a consequence of interactions between the photons and absorbing particles, the power of the beam is attenuated from Po to P. Beer’s law can be written
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
biocapacity → biokapacitet
Biocapacity (or biological capacity) is the capacity of ecosystems to produce useful biological materials and to absorb carbon dioxide generated by humans, using current management schemes and extraction technologies. Useful biological materials are defined as those used by the human economy, hence what is considered useful can change from year to year. The biocapacity of an area is calculated by multiplying the actual physical area by the yield factor and the appropriate equivalence factor.
Yield factor is a factor that accounts for differences between countries in productivity of a given land type. Each country and each year has yield factors for cropland, grazing land, forest, and fisheries.
Equivalence factor is a productivity based scaling factor that converts a specific land type into a universal unit of biologically productive area, a global hectare (gha).
Bohr atom → Bohrov atom
Bohr atom is a model of the atom that explains emission and absorption of radiation as transitions between stationary electronic states in which the electron orbits the nucleus at a definite distance. The Bohr model violates the Heisenberg uncertainty principle since it postulates definite paths and moment for electrons as they move around the nucleus. Modern theories usually use atomic orbitals to describe the behaviour of electrons in atoms.
infrared spectroscopy → infracrvena spektroskopija
Infrared (IR) spectroscopy is a technique used for determining the structure (and sometimes concentration) of molecules by observing how infrared radiation is absorbed by a sample.
Peltier effect → Peltierov efekt
Peltier effect is the absorption or generation of heat (depending on the current direction) which occurs when an electric current is passed through a junction between two materials.
radiography → radiografija
Radiography is nondestructive method of internal examination in which metal objects are exposed to a beam of X-ray or gamma radiation. Differences in thickness, density, or absorption caused by internal defects or inclusions are apparent in the shadow image produced on a fluorescent screen or photographic film placed behind the object.
carbohydrate → ugljikohidrat
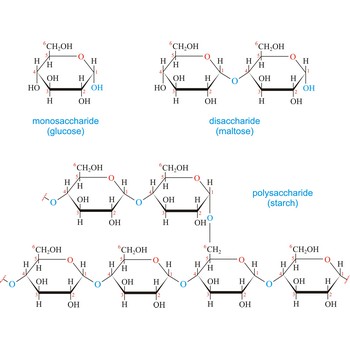
Carbohydrates (often called carbs for short) are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis. They are also known as saccharides, a term derived from the Latin word saccharum for sugar. Carbohydrates are the most abundant class of compounds in the biological world, making up more than 50 % of the dry weight of the Earth’s biomass. Every type of food we eat can have its energy traced back to a plant. Plants use carbon dioxide and water to make glucose, a simple sugar, in photosynthesis. Other carbohydrates such as cellulose and starch are made from the glucose. Light from the sun is absorbed by chlorophyll and this is converted to the energy necessary to biosynthesize carbohydrates
The term carbohydrate was applied originally to monosaccharides, in recognition of the fact that their empirical composition can be expressed as Cx(H2O)y. Later structural studies revealed that these compounds were not hydrates but the term carbohydrate persists.
Carbohydrates are generally classed as either simple or complex. Simple sugars, or monosaccharides, are carbohydrates that can’t be converted into smaller subunits by hydrolysis. Complex carbohydrates are made of two (disaccharides) or more (oligosaccharides, polysaccharides) simple sugars linked together by acetal (glycosidic) bonds and can be split into the former by hydrolysis.
diatomaceous earth → dijatomejska zemlja
Diatomaceous earth is a naturally occurring siliceous sedimentary mineral compound from microscopic skeletal remains (frustules) of diatoms, unicellular aquatic plants of microscopic size. Their fossilized remains are called diatomite and contains approximately 3000 diatom frustules per cubic millimetre.
Diatomite is relatively inert and has a high absorptive capacity, large surface area, and low bulk density. It consists of approximately 90 % silica, and the remainder consists of compounds such as aluminum and iron oxides. The fine pores in the diatom frustules make diatomite an excellent filtering material for waters, beverages, oils, chemicals, as well as many other products.
Citing this page:
Generalic, Eni. "Apsorpcija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table