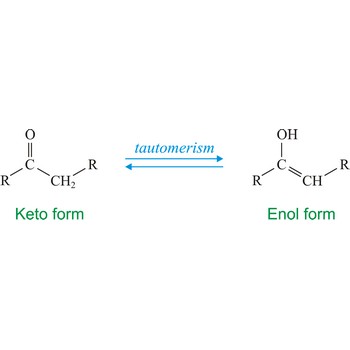
tautomerism → tautomerija
Tautomerism refers to equilibrium between two different structures of the same compound. Usually the tautomers differ in the point of attachment of a hydrogen atom. One of the most common examples of a tautomeric system is the equilibrium between a ketone (keto) and aldehyde (enol).
tear gas → suzavac
Tear gases is the common name for substances which, in low concentrations, cause pain in the eyes, flow of tears and difficulty in keeping the eyes open. Tear gases are used mainly in military exercises and in riot control, etc., but have also been used as a method of warfare. Irritating gases have been used in war since ancient times but it was not until after the Second World War that a more systematic search for effective substances was started. Among a long series of substances, three have become of greater importance than the others. These substances are chloroacetophenone (codename CN), orto-chlorobenzylidene-malononitrile (CS) and dibenz(b,f)-1,4-oxazepine (CR).
thermal resistance → toplinski otpor
Heat always flows from a higher to a lower temperature level. The driving force for the heat flux lies in the temperature difference ΔT between two temperature levels. Analogous to Ohm’s law, the following holds:
where H = dQ/dt is heat flux, measured in watts, ΔT is temperature difference across the thermal resistance, measured in kelvin, and Rth is thermal resistance, measured in K/W.
For example, suppose there were two houses with walls of equal thickness; one is made of glass and the other of asbestos. On a cold day, heat would pass through the glass house much faster. The thermal restistance of asbestos is then higher than of glass.
If the thermal Ohm’s law is divided by the heat capacity C, Newton’s law of cooling is obtained:
where dT/dt is rate of cooling or heating, measured in K s-1, and C is heat capacity, measured in J K-1.
uranium → uranij
Uranium was discovered by Martin Heinrich Klaproth (Germany) in 1789. Named after the planet Uranus. It is silvery-white, dense, ductile, malleable, radioactive metal. Resists alkalis; tarnishes in air; attacked by steam and acids. Radiotoxic. Uranium occurs in many rocks, but in large amounts only in such minerals as pitchblende and carnotite. For many centuries it was used as a pigment for glass. Now it is used as a fuel in nuclear reactors and in bombs.
visible radiation → vidljivo zračenje
Human eye can only see electromagnetic radiation of wavelengths form 400 nm to 760 nm. This narrow part of electromagnetic spectrum is called visible radiation. Visible (white) light is a mixture of light of all kind of colours, it can be separated, with the help of a glass prism, into its component colours - visible light spectrum, and each colour corresponds to a certain area of wavelengths:
| Colour | Wavelength / nm |
|---|---|
| purple | 400 - 450 |
| blue | 450 - 500 |
| green | 500 - 570 |
| yellow | 570 - 590 |
| orange | 590 - 620 |
| red | 620 - 760 |
Ziegler process → Zieglerov proces
Ziegler process is an industrial process for the manufacture of high-density polyethene using catalysts of titanium(IV) chloride (TiCl4) and aluminium alkyls (e.g. triethylaluminium, Al(C2H5)3). The process was introduced in 1953 by the German chemist Karl Ziegler (1898-1973). It allowed the manufacture of polythene at lower temperatures (about 60 °C) and pressures (about 1 atm) than used in the original process.
zirconium → cirkonij
Zirconium was discovered by Martin Heinrich Klaproth (Germany) in 1789. The origin of the name comes from the Arabic word zargun meaning gold colour. It is grey-white, lustrous, corrosion-resistant metal. Exposed surfaces form oxide protective film. Zirconium is found in many minerals such as zircon and baddeleyite. Used in alloys such as zircaloy this is used in nuclear applications since it does not readily absorb neutrons. Also baddeleyite is used in lab crucibles. Used in high-performance pumps and valves. Clear zircon (ZrSiO4) is a popular gemstone.
Schrotter apparatus for determination of CO2 → Schrotterova aparatura za određivanje CO2
Schrötter decomposition apparatus (Schrötter's alkalimeter) is used to determining the carbonate content in samples of limestone, gypsum, dolomite, or baking powder by loss of weight. The apparatus is named after the Austrian chemist Anton Schrötter von Kristelli (1802-1875), who devised it in 1871. The size of the filled apparatus (apparatus is 16 cm high) is such that it weights less than 75 g, and can be placed on the pan of an analytical balance.
Procedure: Weigh about 0.5 g of the powdered carbonate sample and introduce it into the decomposition flask C. Pour into the drying tube A 2-3 mL of concentrated sulphuric acid (H2SO4), and to the dropping funnel B add about 10-15 mL of hydrochloric acid (w(HCl) = 15 %). Weigh the whole apparatus. Open the upper taps of both parts and allow the hydrochloric acid from B to run slowly down on to the powdered sample. The evolved CO2 escapes through the strong sulphuric acid and is thus thoroughly dried. When further addition of acid produces no more evolution of CO2, warm the apparatus up to 80 °C so as to expel the CO2 from the solution. Connect the upper tap of the drying tube A to a water pump and draw a slow current of air through the apparatus until completely cool. Open the upper taps for a moment to equalize the internal and external pressure and weight the apparatus again. The weight loss is equal to the weight of carbon dioxide liberated from the carbonates.
Citing this page:
Generalic, Eni. "Alicikli�ki spojevi." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table