transition metal → prijelazni element
This group of metals is distinguished from other metals not by their physical properties, but by their electronic structure. Transition metals are elements characterized by a partially filled d subshell. The First Transition Series comprises scandium (Sc), titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni) and copper (Cu). The Second and Third Transition Series include the lanthanides and actinides, respectively.
The transition metals are noted for their variability in oxidation state. Thus, manganese has two electrons in its outside shell and five electrons in the next shell down, and exhibits oxidation states of +1, +2, +3, +4, +5, +6, and +7.
They are also characterised by the fact that well into the series, going from left to right, the properties of the succeeding metals do not differ greatly from the preceding ones.
triols → trioli
Trihydric alcohols (i.e. Triols) are organic compounds containing three hydroxyl groups. The simplest trihydric alcohol is 1,2,3-propane-triol, CH2(OH)CH(OH)CH2(OH), which is also known as glycerol (from the Greek glykys meaning sweet) or glycerin. Glycerol is commercially produced by the hydrolysis of fats.
Glycerol is a by-product in the soap industry and is recovered by suitable means.
uranium → uranij
Uranium was discovered by Martin Heinrich Klaproth (Germany) in 1789. Named after the planet Uranus. It is silvery-white, dense, ductile, malleable, radioactive metal. Resists alkalis; tarnishes in air; attacked by steam and acids. Radiotoxic. Uranium occurs in many rocks, but in large amounts only in such minerals as pitchblende and carnotite. For many centuries it was used as a pigment for glass. Now it is used as a fuel in nuclear reactors and in bombs.
vanadium → vanadij
Vanadium was discovered by A. M. del Rio (Spain) in 1801 and rediscovered by Nils Sefstrom (Sweden) in 1830. Named after Vanadis, the goddess of beauty in Scandinavian mythology. It is soft, ductile, silvery-white metal. Resistant to corrosion by moisture, air and most acids and alkalis at room temperature. Exposed surfaces form oxide coating. Reacts with concentrated acids. Vanadium is found in the minerals patronite (VS4), vanadinite [Pb5(VO4)3Cl] and carnotite [K2(UO2)2(VO4)2·3H2O]. Pure metal produced by heating with C and Cl to produce VCl3 which is heated with Mg in Ar atmosphere. It is mixed with other metals to make very strong and durable alloys. Vanadium pentoxide (V2O5) is used as a catalyst, dye and fixer-fixer.
water gas → vodeni plin
Water gas (blue gas, synthesis gas) is a fuel gas used in industrial synthesis of organic chemicals, and in welding, glassmaking, and other high-temperature industrial applications. Water gas is made by passing steam over a bed of hot coal or coke. It mainly consists of carbon monoxide (CO) and hydrogen (H2), contaminated with small amounts of CO2, N2, CH4, and O2.
water hardness → tvrdoća vode
Hardness is defined as the concentrations of calcium and magnesium ions expressed in terms of calcium carbonate. These minerals in water can cause some everyday problems. They react with soap and produce a deposit called soap curd that remains on the skin and clothes and, because it is insoluble and sticky, cannot be removed by rinsing.
Hard water may also shorten the life of plumbing and water heaters. When water containing calcium carbonate is heated, a hard scale is formed that can plug pipes and coat heating elements. Scale is also a poor heat conductor. With increased deposits on the unit, heat is not transmitted to the water fast enough and overheating of the metal causes failure. Build-up of deposits will also reduce the efficiency of the heating unit, increasing the cost of fuel.
There are two types of water hardness, temporary and permanent.
Temporary Hardness is due to the bicarbonate ion, HCO3-, being present in the water. This type of hardness can be removed by boiling the water to expel the CO2, as indicated by the following equation:
Permanent hardness is due to calcium and magnesium nitrates, sulphates, and chlorides etc. This type of hardness cannot be eliminated by boiling.
| Water supply classification | |
|---|---|
| Hardness | Concentration of Calcium carbonate (mg/L) |
| Soft Water | 0 to 75 |
| Medium Hard Water | 75 to 150 |
| Hard Water | 150 to 300 |
| Very Hard Water | over 300 |
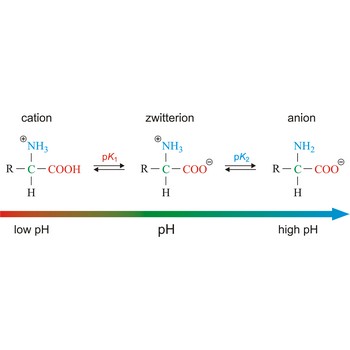
zwitterion → dipolarni ion
Zwitterion, also known as inner salt or dipolar ion, is an ion with a positive and a negative electrical charge at different locations within a molecule. As the molecule contains two opposite charges, it is electrically neutral. The term zwitterion is derived from the German word zwitter, meaning a hybrid, hermaphrodite. Zwitterions can be formed from compounds that contain both acid groups and base groups in their molecules (ampholytes).
All of the common amino acids found in proteins are ampholytes because they contain a carboxyl group (-COOH) that acts as an acid and an amino group (-NH2) that acts as a base. In the solid state, amino acids exist in the dipolar or zwitterion form. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (H+) ion, and the amino acid becomes positively charged. If base is added, ion removal of the H+ ion from the amino group of the zwitterion produces a negatively charged amino acid.
Citing this page:
Generalic, Eni. "Aliciklički spojevi." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table