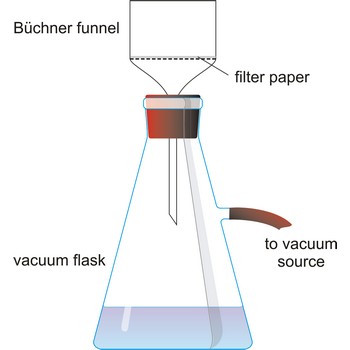
filter flask → boca za odsisavanje
Filter flask, also known as a vacuum flask, is a flask with a side arm to which a vacuum can be applied. It usually have heavy side walls to withstand high vacuum.
foam → pjena
Foams are dispersions of gases in liquids or solids. The gas globule may be of any size, from colloidal to macroscopic, as in soap bubbles. Bakers’ bread and sponge rubber are examples of solid foams. Typical liquid foams are those used in fire-fighting, shaving creams, etc. Foams made by mechanical incorporation of air are widely used in the food industry (e.g. whipped cream, egg white, ice cream, etc.). Foams can be stabilized by surfactants.
refractory metal → vatrostalni metal
Refractory metal is a metal having an extremely high melting point, for example tungsten, molybdenum, niobium, tantalum, and rhenium
reverse osmosis → reverzna osmoza
Reverse osmosis is the method used for obtaining freshwater from saltwater. The process uses a semi-permeable membrane through which pure water and not the salts will pass. The saltwater must be pressurised to approximately 25 bar, which makes it operationally expensive to produce large quantities of fresh water by this method.
fossil fuel → fosilno gorivo
Fossil fuels (coal, oil, and natural gas) are the fuels used by man as a source of energy. They are formed from the remains of living organisms and all have a high carbon or hydrogen content. They have value as fuels on the exothermic oxidation of carbon to form carbon dioxide
and the oxidation of hydrogen to form water
Frasch proces → Fraschov postupak
Frasch proces is a method of obtaining sulphur from underground deposits using a tube consisting of three concentric pipes. Superheated steam is passed down the outer pipe to melt the sulphur, which is forced up through the middle pipe by compressed air fed through the inner tube. The steam in the outer casing keeps the sulphur molten in the pipe. It was named after the German-born American chemist Herman Frasch (1851-1914).
Froude number → Froudova značajka
Froude number (Fr) is a dimensionless quantity used in fluid mechanics, defined by
where v is velocity, l is length, and g is acceleration due to gravity.
fuel cell → gorivi članak
Fuel cell is a device that converts chemical energy into electrical energy. It is different from a battery in that the energy conversion continues as long as fuel and oxidising agent are fed to the fuel cell; that is, in principle indefinitely. (A battery is manufactured with a limited amount of chemicals, and it is exhausted when all the chemicals have reacted.) It is a galvanic cell where spontaneous chemical reactions occur at the electrodes. The fuel is oxidised at the anode, and the oxidising agent (almost always oxygen or air) is reduced at the cathode. Presently, the most commonly used fuel is hydrogen. More conventional fuels (e.g., petrol or natural gas) must be converted (reformed) into hydrogen before they can be utilised in a fuel cell.
Some fuel cells employ an aqueous solution as electrolyte, that can be either acidic or basic (alkaline), or an ion-exchange membrane soaked in aqueous solution can act as the electrolyte. These fuel cells operate at relatively low temperatures (from room temperature to not much above the boiling point of water). Some fuel cells employ molten salts (especially carbonates) as electrolytes and have to operate at temperatures of several hundred degrees centigrade (Celsius). Others employ ionically conductive solids as electrolyte and must operate close to 1 000 °C.
Citing this page:
Generalic, Eni. "Zemljina kora." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table