Results 1–8 of 8 for YARIS MODELS
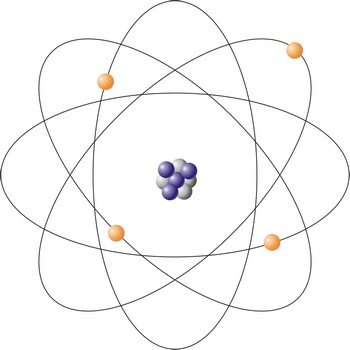
atom → atom
Atom is an atom is the smallest particle of an element that retains the chemical properties of the element. Rutherford-Bohr’s model represents the atom as a positively charged core of a size around 10-14 m composed of protons (positive particles) and neutrons (neutral particles) around which negatively charged electrons circle. The number of protons and electrons are equal, so the atom is an electrically a neutral particle. Diameter of the atom is about 10-10 m.
Bohr atom → Bohrov atom
Bohr atom is a model of the atom that explains emission and absorption of radiation as transitions between stationary electronic states in which the electron orbits the nucleus at a definite distance. The Bohr model violates the Heisenberg uncertainty principle since it postulates definite paths and moment for electrons as they move around the nucleus. Modern theories usually use atomic orbitals to describe the behaviour of electrons in atoms.
borane → borani
Borane is any of the group of compounds of boron and hydrogen (B2H6, B4H10, B5H9, B5H11...), many of which can be prepared by action of acid on magnesium boride (Mg3B2). Boranes are a remarkable group of compounds in that their structures cannot be described using the conventional two-electron covalent bond model.
electrical double layer → električni dvosloj
Electrical double layer is the structure of charge accumulation and charge separation that always occurs at the interface when an electrode is immersed into an electrolyte solution. The excess charge on the electrode surface is compensated by an accumulation of excess ions of the opposite charge in the solution. The amount of charge is a function of the electrode potential. This structure behaves essentially as a capacitor. There are several theoretical models that describe the structure of the double layer. The three most commonly used ones are the Helmholtz model, the Gouy-Chapman model, and the Gouy-Chapman-Stern model.
Newman’s projection → Newmanova projekcija
Newman’s projection is an image which we get when we observe a model of ethane molecule in C-C bond direction.
Lennard-Jones potential → Lennard-Jonesov potencijal
The Lennard-Jones potential (or 12-6 potential) is a mathematically simple model that describes the interaction between two non-bonded and uncharged atoms (known as the van der Waals interaction). It was first proposed in 1924 by British physicist Sir John Edward Lennard-Jones (1894-1954). The Lennard-Jones Potential is given by the following equation
V(r) = 4e[(sigma/r)12-(sigma/r)6)]
where V is the intermolecular potential between the two atoms or molecules, ε is the well depth and a measure of how strongly the two particles attract each other, σ is the distance at which the intermolecular potential between the two particles is zero, r is the distance of separation between centres of both particles.
Citing this page:
Generalic, Eni. "YARIS MODELS." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table