titanium → titanij
Titanium was discovered by William Gregor (England) in 1791. Named after the Titans, the sons of the Earth goddess in Greek mythology. It is shiny, dark-grey metal. Powdered form burns in air. Exposed surfaces form oxide coating. It can be highly polished and is relatively immune to tarnishing. Unreactive with alkali and most acids. Titanium usually occurs in the minerals ilmenite (FeTiO3), rutile (TiO2) and iron ores. Pure metal produced by heating TiO2 with C and Cl2 to produce TiCl4 then heated with Mg gas in Ar atmosphere. Since it is strong and resists acids it is used in many alloys. Titanium dioxide (TiO2), a white pigment that covers surfaces very well, is used in paint, rubber, paper and many others.
tungsten → volfram
Tungsten was discovered by Fausto and Juan Jose de Elhuyar (Spain) in 1783. Named after the tungsten mineral wolframite. It is hard, steel-grey to white metal. Highest melting point of all metals. Resists oxygen, acids and alkalis. Tungsten occurs in the minerals scheelite (CaWO4) and wolframite [(Fe,Mn)WO4]. Made into filaments for vacuum tubes and electric lights. Also as contact points in cars. Tungsten carbide is extremely hard and is used for making cutting tools and abrasives.
U-tube manometer → U-manometar
U-tube manometer contains water or mercury in a U-shaped tube, and is usually used to measure gas pressure. One end of the U tube is exposed to the unknown pressure field (P) and the other end is connected to a reference pressure source (usually atmospheric pressure) (Pref), shown in the schematic below.
If fluid C is the atmosphere, fluid B is the liquid in the U tube (e.g. water or mercury), and fluid A is a gas, then we can assume that ρB >> ρA, ρC. The pressure contributed by the weight of gas within the U tube can therefore be neglected. The gage pressure of the gas can be approximated by,
ultraviolet light → ultraljubičasto svjetlo
Ultraviolet light (UV light or UV radiation) is an electromagnetic radiation with a wavelength longer than that of x-rays, but shorter than that of visible light. Ultraviolet light can break some chemical bonds and cause cell damage.
uranium → uranij
Uranium was discovered by Martin Heinrich Klaproth (Germany) in 1789. Named after the planet Uranus. It is silvery-white, dense, ductile, malleable, radioactive metal. Resists alkalis; tarnishes in air; attacked by steam and acids. Radiotoxic. Uranium occurs in many rocks, but in large amounts only in such minerals as pitchblende and carnotite. For many centuries it was used as a pigment for glass. Now it is used as a fuel in nuclear reactors and in bombs.
Wilson’s chamber → Wilsonova komora
Wilson’s chamber is used for detection of radioactive radiation. Wilson’s chamber has a glass cylinder filled with air that has been saturated with water vapour. Radioactive radiation in its way ionises molecules of gas which then function as centres on which water vapour condenses into very small drops, thereupon showing Tyndall’s effect, i.e. is they are visible as a bright trail.
X-ray diffraction → rendgenska difrakcija
The regular array of atoms in a crystal is a three-dimensional diffraction grating for short-wavelength waves such as X-rays. The atoms are arranged in planes with interplanar spacing d. Diffraction maxima occur in the incident direction of the wave, measured from the surface of a plane of atoms, and the wavelength λ of the radiation satisfy Braggs’s law:
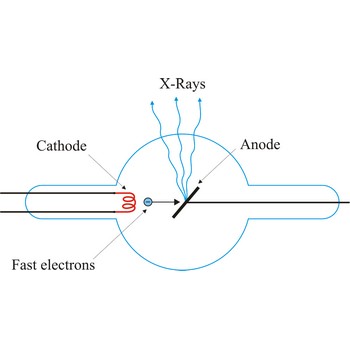
X-ray tube → rendgenska cijev
X-ray tube is a cathode ray tube that focuses energetic streams of electrons on a metal target, causing the metal to emit X-rays. The basic principle of the X-ray tube has not changed significantly since Roentgen's 1895 discovery. Current applied to a metal cathode (about 50 000 V) produces free electrons. The X-rays are produced when the rapidly moving electrons are suddenly stopped as they strike the metal target of the tube.
xenon → ksenon
Xenon was discovered by Sir William Ramsay, Morris W. Travers (England) in 1898. The origin of the name comes from the Greek word xenos meaning stranger. It is heavy, colourless, odourless, noble gas. Reacts only with fluorine. Xenon is obtain from the small quantities in liquid air. Used for filling flash lamps and other powerful lamps. Electrical excitation of xenon produces a burst of brilliant white light. Also used in bubble chambers and modern nuclear power reactors.
ytterbium → iterbij
Ytterbium was discovered by Jean de Marignac (France) in 1878. Named after Ytterby, a village in Sweden. It is silvery, lustrous, malleable and ductile metal. Oxidizes slowly in air. Reacts with water. Flammable dust. Ytterbium is found in minerals such as yttria, monazite, gadolinite and xenotime. Used in metallurgical and chemical experiments.
Citing this page:
Generalic, Eni. "X-zrake." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table