lutetium → lutecij
Lutetium was discovered by Georges Urbain (France) and independently by Carl Auer von Welsbach (Austria) in 1907. The origin of the name comes from the Greek word Lutetia meaning Paris. It is silvery-white and relatively stable in air, rare earth metal. Lutetium is found with ytterbium in gadolinite and xenotime. Stable lutetium nuclides can be used as catalysts in cracking, alkylation, hydrogenation, and polymerization.
magnesium → magnezij
Magnesium was discovered by Sir Humphry Davy (England) in 1808. The origin of the name comes from the Greek word Magnesia, a district of Thessaly. It is lightweight, malleable, silvery-white metal. Burns in air with a brilliant white flame and reacts with water as temperature elevates. Can ignite in air. React violently with oxidants. Magnesium is found in large deposits in the form of magnesite, dolomite and other minerals. It is usually obtained by electrolysis of melted magnesium chloride (MgCl2) derived from brines, wells and sea water. Used in alloys to make airplanes, missiles and other uses for light metals. Have structural properties similar to aluminium.
mercury → živa
Mercury has been known since ancient times. The origin of the name comes from the Latin word hydrargyrum meaning liquid silver. It is heavy, silver-white metal, liquid at ordinary temperatures. Stable in air and water. Unreactive with alkalis and most acids. Gives off poisonous vapour. Chronic cumulative effects. Mercury only rarely occurs free in nature. The chief ore is cinnabar or mercury sulfide (HgS). Used in thermometers, barometers and batteries. Also used in electrical switches and mercury-vapour lighting products.
neodymium → neodimij
Neodymium was discovered by Carl F. Auer von Welsbach (Austria) in 1885. The origin of the name comes from the Greek words neos didymos meaning new twin. It is silvery-white, rare-earth metal that oxidizes easily in air. Reacts slowly in cold water, more rapidly as heated. Metal ignites and burns readily. Neodymium is made from electrolysis of its halide salts, which are made from monazite sand. Used in making artificial ruby for lasers. Also in ceramics and for a special lens with praseodymium. Also to produce bright purple glass and special glass that filters infrared radiation. Misch metal, used in the manufacture of pyrophoric alloys for cigarette lighters, contains about 18 % neodymium metal. (Typically composition of misch metal are Ce:Nd:Pr:La:Other rare earth=50:18:6:22:4). Neodymium is used to create some of the most powerful permanent magnets on Earth, known as NIB magnets they consist of neodymium, iron, and boron.
nickel → nikal
Nickel was discovered by Axel Fredrik Cronstedt (Sweden) in 1751. The origin of the name comes from the German word kupfernickel meaning Devil’s copper or St Nicholas’s (Old Nick’s) copper. It is hard, malleable, silvery-white metal. Soluble in acids, resist alkalis. It can be polished to a lustrous finish. Resists corrosion in air under normal conditions. Nickel is chiefly found in pentlandite [(Ni,Fe)9S8] ore. The metal is produced by heating the ore in a blast furnace which replaces the sulfur with oxygen. The oxides are then treated with an acid that reacts with the iron not the nickel. Used in electroplating and metal alloys because of its resistance to corrosion. Also in nickel-cadmium batteries, as a catalyst and for coins.
nitrogen → dušik
Nitrogen was discovered by Daniel Rutherford (Scotland) in 1772. The origin of the name comes from the Greek words nitron genes meaning nitre and forming and the Latin word nitrum (nitre is a common name for potassium nitrate, KNO3). It is colourless, odourless, generally inert gas. Minimally reactive at room temperature. A component of many organic and inorganic compounds. Makes up about 78 % of earth’s atmosphere. Nitrogen is obtained from liquid air by fractional distillation. Primarily to produce ammonia and other fertilizers. Also used in making nitric acid, which is used in explosives. Also used in welding and enhanced oil recovery.
osmium → osmij
Osmium was discovered by Smithson Tennant (England) in 1803. The origin of the name comes from the Greek word osme meaning smell. It is hard fine black powder or hard, lustrous, blue-white metal. Unaffected by air, water and acids. Characteristic acrid, chlorine like odour due to tetroxide compound. Osmium tetroxide highly toxic. Osmium is obtained from the same ores as platinum. Used to tip gold pen points, instrument pivots, to make electric light filaments. Used for high temperature alloys and pressure bearings. Very hard and resists corrosion better than any other.
Ostwald’s process → Ostwaldov proces
Ostwald’s process is a process by which the nitric acid can be obtained in three degrees. In the first stage ammonia and oxygen react (with platinum-rhodium as a catalyst), whereby the nitrogen monoxide and water emerge
In the second stage nitrogen monoxide reacts with oxygen whereby nitrogen dioxide emerges
and in the third stage nitrogen dioxide dissolves in water, in the presence of air, giving the nitric acid
oxygen → kisik
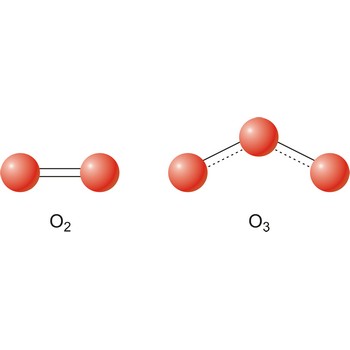
Oxygen was discovered by Joseph Priestley (England) in 1774. The origin of the name comes from the Greek words oxy genes meaning acid and forming (acid former). It is colourless, odourless gas; pale blue liquid. Extremely reactive. Forms oxides with nearly all other elements except noble gases. It is the most abundant element in the earth’s crust and makes up almost 21 % of the atmosphere. Oxygen is obtained primarily from liquid air by fractional distillation. Small amounts are made in the laboratory by electrolysis of water. Used in steel making, welding and supporting life. Naturally occurring ozone (O3) in the upper atmosphere shields the earth from ultraviolet radiation.
Citing this page:
Generalic, Eni. "X-zrake." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table