metallic glass → metalno staklo
Certain alloys can solidify by extremely rapid cooling out of melt without formation of a crystal lattice, that is in the amorphous form - such, amorphous alloys are so called metallic glasses. The alloy of zirconium, beryllium, titanium, copper, and nickel is one of the first metallic glasses that can be made in bulk and formed into strong, hard, useful objects.
Unlike pure metals and most metal alloys, metallic glasses have no regular crystalline structure. This lack of long range order or microstructure is related to such desirable features as strength and low damping which is one reason why the premier use for zirconium-based metallic glass is in the manufacture of expensive golf club heads. Metallic glasses can be quite strong yet highly elastic, and they can also be quite tough (resistant to fracture). Even more interesting are the thermal properties; for instance, just like an oxide glass, there is a temperature (called the glass transition temperature) above which a metallic glass becomes quite soft and flows easily. This means that there are lots of opportunities for easily forming metallic glasses into complex shapes.
potentiometric titration → potenciometrijska titracija
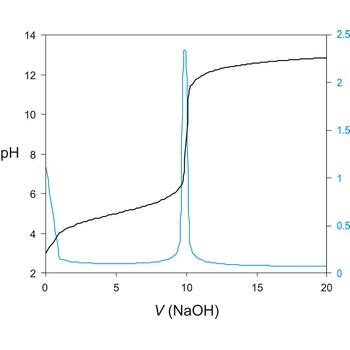
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
qualitative analysis → kvalitativna analiza
Qualitative analysis involves determining the nature of a pure unknown compound or the compounds present in a mixture. Qualitative inorganic analysis is used to separate and detect cations and anions in a sample substance. According to their properties, cations are usually classified into six groups. Each group has a common reagent which can be used to separate them from the solution.
salinity → salinitet
Salinity (S) is a measure of the quantity of dissolved salts in seawater. It is formally defined as the total amount of dissolved solids in seawater in parts per thousand (‰) by weight when all the carbonate has been converted to oxide, the bromide and iodide to chloride, and all organic matter is completely oxidized.
Chlorinity is the oldest of the salinity measures considered and is still a corner-stone in the study of dissolved material in seawater. Based on the principle of constant relative proportions it provides a measure of the total amount of dissolved material in seawater in terms of the concentration of halides. The relationship between chlorinity (Cl) and salinity as set forth in Knudsen’s tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
Practical Salinity (SP) was introduced as a replacement for Chlorinity. Practical Salinity is is relatively easy to measure using standard conductometers, measurements are more precise and less time consuming than measurements of Chlorinity and accurate measurements can even be made in situ. Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution).
Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". For most purposes one can assume that the psu and the ‰, are synonymous.
The global average salinity of ocean waters is about 35 ‰, that is, about 35 g of solid substances are dissolved in 1 kg of seawater.
Soxhlet extractor → Soxhletov ekstraktor
Soxhlet extractor is a laboratory apparatus designed to extract substances with a low solubility in the extracting solvent. The method described by the German chemist Franz von Soxhlet (1848-1926) in 1879 is the most commonly used example of a semi-continuous method applied to extraction of lipids from foods. In the Soxhlet extractor, the sample soaks in hot solvent that is periodically siphoned off, distilled and returned to the sample. During each cycle, a portion of the non-volatile compound dissolves in the solvent. After many cycles the desired compound is concentrated in the distillation flask. The solvent in the flask is then evaporated and the mass of the remaining lipid is measured.
standard → standard
Standards are materials containing a known concentration of an analyte. They provide a reference to determine unknown concentrations or to calibrate analytical instruments.
The accuracy of an analytical measurement is how close a result comes to the true value. Determining the accuracy of a measurement usually requires calibration of the analytical method with a known standard. This is often done with standards of several concentrations to make a calibration or working curve.
A primary standard is a reagent that is extremely pure, stable, has no waters of hydration, and has a high molecular weight.
A secondary standard is a standard that is prepared in the laboratory for a specific analysis. It is usually standardised against a primary standard.
Schrotter apparatus for determination of CO2 → Schrotterova aparatura za određivanje CO2
Schrötter decomposition apparatus (Schrötter's alkalimeter) is used to determining the carbonate content in samples of limestone, gypsum, dolomite, or baking powder by loss of weight. The apparatus is named after the Austrian chemist Anton Schrötter von Kristelli (1802-1875), who devised it in 1871. The size of the filled apparatus (apparatus is 16 cm high) is such that it weights less than 75 g, and can be placed on the pan of an analytical balance.
Procedure: Weigh about 0.5 g of the powdered carbonate sample and introduce it into the decomposition flask C. Pour into the drying tube A 2-3 mL of concentrated sulphuric acid (H2SO4), and to the dropping funnel B add about 10-15 mL of hydrochloric acid (w(HCl) = 15 %). Weigh the whole apparatus. Open the upper taps of both parts and allow the hydrochloric acid from B to run slowly down on to the powdered sample. The evolved CO2 escapes through the strong sulphuric acid and is thus thoroughly dried. When further addition of acid produces no more evolution of CO2, warm the apparatus up to 80 °C so as to expel the CO2 from the solution. Connect the upper tap of the drying tube A to a water pump and draw a slow current of air through the apparatus until completely cool. Open the upper taps for a moment to equalize the internal and external pressure and weight the apparatus again. The weight loss is equal to the weight of carbon dioxide liberated from the carbonates.
Citing this page:
Generalic, Eni. "Winklerova metoda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table