proton → proton
Proton is a stable elementary particle of unit positive charge and spin 1/2. Protons and neutrons, which are collectively called nucleons, are the constituents of the nucleus.
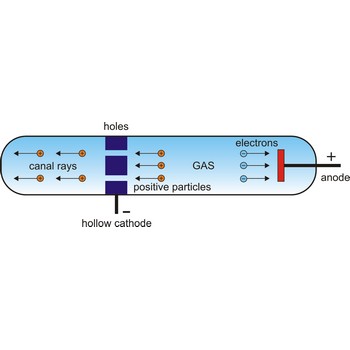
In 1886, German physicist Eugene Goldstein (1850-1930) discovered positive particles by using a modified Crookes tube with holes in the cathode in an evacuated tube. When cathode rays were given off in one direction toward the anode, other rays found their way through the holes in the cathode and sped off in the opposite direction. Since these other rays traveled in the direction opposite to the negatively charged cathode rays, it seemed that they must be composed of positively charged particles. Rutherford suggested that this fundamental positive particle be called the proton.
rubidium → rubidij
Rubidium was discovered by Robert Bunsen and Gustav Kirchhoff (Germany) in 1861. The origin of the name comes from the Latin word rubidius meaning dark red or deepest red. It is soft, silvery-white, highly reactive metal. Ignites in air. Reacts violently with water or oxidants. Rubidium occurs abundantly, but so widespread that production is limited. Usually obtained from lithium production. Used as a catalyst, photocells and vacuum and cathode-ray tubes.
Torricelli, Evangelista → Torricelli, Evangelista
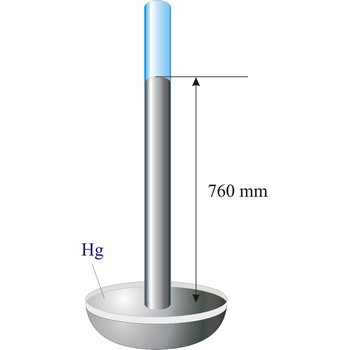
Evangelista Torricelli (1852-1908) is Italian physicist and mathematician. He became the first scientist to create a sustained vacuum and to discover the principle of a barometer. He filled a tube three feet long, and hermetically closed at one end, with mercury and set it vertically with the open end in a basin of mercury, taking care that no air-bubbles should get into the tube. The column of mercury invariably fell to about twenty-eight inches, leaving an empty space above its level. He discovered that the variation of the height of the mercury from day to day was caused by changes in the atmospheric pressure. He also constructed a number of large objectives and small, short focus, simple microscopes.
tungsten → volfram
Tungsten was discovered by Fausto and Juan Jose de Elhuyar (Spain) in 1783. Named after the tungsten mineral wolframite. It is hard, steel-grey to white metal. Highest melting point of all metals. Resists oxygen, acids and alkalis. Tungsten occurs in the minerals scheelite (CaWO4) and wolframite [(Fe,Mn)WO4]. Made into filaments for vacuum tubes and electric lights. Also as contact points in cars. Tungsten carbide is extremely hard and is used for making cutting tools and abrasives.
volumetric pipette → prijenosna pipeta
Volumetric pipettes (transfer or belly pipette) are used in volumetric analysis, when there is a need for taking exact smaller volume of a sample solution or reagent. The upper tube of volumetric pipette has a ringlike marking (mark) which marks its calibrated volume. Pipettes calibrated to deliver (TD or Ex) the indicated volume. By sucking in (with mouth, propipette or a water pump) the liquid is pulled in a little bit above the mark and the opening of the pipet is closed with a forefingertip. Outer wall of pipet is wiped and, with a slight forefinger loosening, the liquid is released until it reaches the mark. Mark must figure as a tangent on a lower edge of the liquid meniscus. A pipette is emptied out by lifting the forefinger off and letting the liquid flow out of the pipette freely. After another 15 s and the tip of the pipette is pulled onto the inner wall of the vessel. It is absolutely forbidden to blow out the contents of the pipette.
wash bottle → boca štrcaljka
Plastic wash bottle is a squeeze bottle made of low density polyethylene (LDPE) whose contents can be forced out through a narrow hole at the top by squeezing the bottle.
Glass wash bottle is a bottle fitted with two glass tubes pass through the cap, so that on blowing into one of the tubes a stream of water issuing from the other may be directed upon anything to be washed or rinsed, as a precipitate upon a filter.
Schrotter apparatus for determination of CO2 → Schrotterova aparatura za određivanje CO2
Schrötter decomposition apparatus (Schrötter's alkalimeter) is used to determining the carbonate content in samples of limestone, gypsum, dolomite, or baking powder by loss of weight. The apparatus is named after the Austrian chemist Anton Schrötter von Kristelli (1802-1875), who devised it in 1871. The size of the filled apparatus (apparatus is 16 cm high) is such that it weights less than 75 g, and can be placed on the pan of an analytical balance.
Procedure: Weigh about 0.5 g of the powdered carbonate sample and introduce it into the decomposition flask C. Pour into the drying tube A 2-3 mL of concentrated sulphuric acid (H2SO4), and to the dropping funnel B add about 10-15 mL of hydrochloric acid (w(HCl) = 15 %). Weigh the whole apparatus. Open the upper taps of both parts and allow the hydrochloric acid from B to run slowly down on to the powdered sample. The evolved CO2 escapes through the strong sulphuric acid and is thus thoroughly dried. When further addition of acid produces no more evolution of CO2, warm the apparatus up to 80 °C so as to expel the CO2 from the solution. Connect the upper tap of the drying tube A to a water pump and draw a slow current of air through the apparatus until completely cool. Open the upper taps for a moment to equalize the internal and external pressure and weight the apparatus again. The weight loss is equal to the weight of carbon dioxide liberated from the carbonates.
Citing this page:
Generalic, Eni. "U-tube." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table