Results 361–370 of 1083 for Startseite Charts Anmelden Tools Schlagw%F6rter Mitgliederbereich language:en
capacitor → kondenzator
Capacitor is a device that stores electric charges. The symbol for a capacitor in electric circuit schemes is —| |—.
carbohydrate → ugljikohidrat
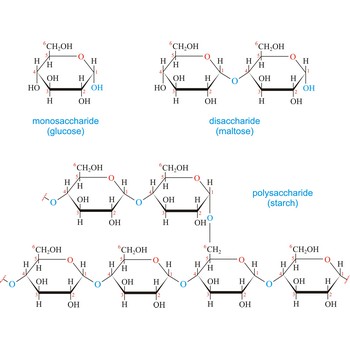
Carbohydrates (often called carbs for short) are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis. They are also known as saccharides, a term derived from the Latin word saccharum for sugar. Carbohydrates are the most abundant class of compounds in the biological world, making up more than 50 % of the dry weight of the Earth’s biomass. Every type of food we eat can have its energy traced back to a plant. Plants use carbon dioxide and water to make glucose, a simple sugar, in photosynthesis. Other carbohydrates such as cellulose and starch are made from the glucose. Light from the sun is absorbed by chlorophyll and this is converted to the energy necessary to biosynthesize carbohydrates
The term carbohydrate was applied originally to monosaccharides, in recognition of the fact that their empirical composition can be expressed as Cx(H2O)y. Later structural studies revealed that these compounds were not hydrates but the term carbohydrate persists.
Carbohydrates are generally classed as either simple or complex. Simple sugars, or monosaccharides, are carbohydrates that can’t be converted into smaller subunits by hydrolysis. Complex carbohydrates are made of two (disaccharides) or more (oligosaccharides, polysaccharides) simple sugars linked together by acetal (glycosidic) bonds and can be split into the former by hydrolysis.
decomposing → raščinjavanje
Decomposing in analytical chemistry means that a certain substance is converted, by melting it with a suitable melting medium (sodium carbonate, sodium hydroxide, sodium peroxide, ...) in the kind of compound which will afterwards that dissolve in water, acid or base very easily.
decomposition potential → potencijal razlaganja
Decomposition potential of some system is the smallest voltage which should be applied so that electrolysis occurs.
degenerate orbitals → degenerirane orbitale
Degenerate orbitals are orbitals with the same energy. This degeneracy can sometimes be "lifted" by external electric or magnetic fields.
dehydrogenation → dehidrogenacija
Dehydrogenation is a chemical reaction in which hydrogen is removed from a compound. Dehydrogenation of organic compounds converts single carbon-carbon bonds into double bonds. It is usually affected by means of a metal catalyst or in biological systems by enzyme dehydrogenases.
diffusion current → difuzijska struja
Diffusion current (id) is a current which is limited by the speed of particle diffusion in an electrolyte solution.
carbon → ugljik
Carbon has been known since ancient times. The origin of the name comes from the Latin word carbo meaning charcoal. Graphite form of carbon is a black, odourless, slippery solid. Graphite sublimes at 3825 °C. Diamond form is a clear or colored; an extremely hard solid. C60 is Buckminsterfullerine. Carbon black burns readily with oxidants. Carbon is made by burning organic compounds with insufficient oxygen. There are close to ten million known carbon compounds, many thousands of which are vital to organic and life processes. Radiocarbon dating uses the carbon-14 isotope to date old objects.
carboxylic acids → karboksilne kiseline
Carboxylic acids are organic compounds characterized by the presence of one or more RC(=O)OH groups (the carboxyl group). In the systematic chemical nomenclature carboxylic acids names end in the suffix -oic (e.g. ethanoic acids, CH3COOH). The carbon of the terminal group being counted as part of the chain. They are generally weak acids. Carboxylic acids include a large and important class of fatty acids and may be either saturated or unsaturated. There are also some natural aromatic carboxylic acids (benzoic, salicylic).
Citing this page:
Generalic, Eni. "Startseite Charts Anmelden Tools Schlagw%F6rter Mitgliederbereich language:en." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table