vacuum distillation → vakuum destilacija
Vacuum distillation is distillation under reduced pressure. The depression in the boiling point of the substance distilled means that the temperature is lower, which may prevent the substance from decomposing.
hexagonal lattice → heksagonska rešetka
Hexagonal lattice has lattice points at the twelve corners of the hexagonal prism and at the centers of the two hexagonal faces of the unit cell. It has unit cell vectors a=b≠c and interaxial angles α=β=90° and γ=120°.
indium → indij
Indium was discovered by Ferdinand Reich and Hieronymus Theodor Richter (Germany) in 1863. Named after the indicum (colour indigo), the colour it shows in a spectroscope. It is rare, very soft, silver-white metal. Stable in air and water. Dissolves in acids. Metal can ignite and burn. Indium is found in certain zinc ores. Used to coat high speed bearings and as an alloy that lowers the melting point of other metals. Relatively small amounts are used in dental items and in electronic semiconductors.
iridium → iridij
Iridium was discovered by Smithson Tennant (England) in 1803. The origin of the name comes from the Latin word iris, meaning rainbow, because its salts are highly colored. It is heavy, brittle, white metal. Unreactive in air, water and acids. Attacked by fused NaOH. Metal ignites and burns readily. Iridium is found in gravel deposits with platinum. Used with osmium to tip gold pen points, to make crucible and special containers. Also to make alloys used for standard weights and measures and heat-resistant alloys. Also as hardening agent for platinum.
volt → volt
Volt (V) is the SI derived unit of electric potential. One volt is the difference of potential between two points of an electric conductor when a current of 1 ampere flowing between those points dissipates a power of 1 watt. It was named after the Italian physicist Alessandro Volta (1745-1827).
kelvin → kelvin
Kelvin (K) is the SI base unit of thermodynamic temperature.
The kelvin, unit of thermodynamic temperature, is the fraction 1/273.16 of the thermodynamic temperature of the triple point of water. The unit was named after the British scientist Sir. W. Thompson, Lord Kelvin (1824-1907).ligand → ligand
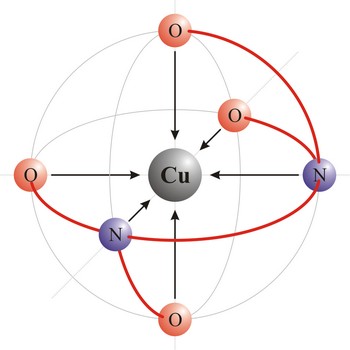
Ligand is an ion (F-, Cl-, Br-, I-, S2-, CN-, NCS-, OH-, NH2-) or molecule (NH3, H2O, NO, CO) that donates a pair of electrons to a metal atom or ion in forming a coordination complex. The main way of classifying ligands is by the number of points at which they are attached to, or bound to, the metal center. This is the denticity. Ligands with one potential donor atom are monodentate. Polydentate ligand is a ligand that is attached to a central metal ion by bonds from two or more donor atoms. Ligands with more than one potential donor atom are known as ambidentate, such as the thiocyanate ion, NCS-, which can bind to the metal center with either the nitrogen or sulphur atoms. Chelating ligands are those polydentate ligands which can form a ring including the metal atom.
Citing this page:
Generalic, Eni. "ON POINT." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table