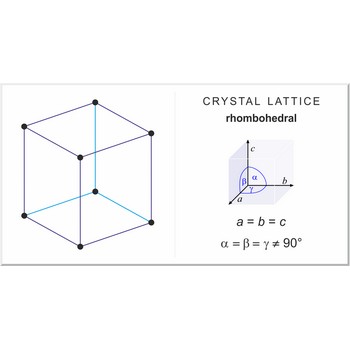
rhombohedral lattice → romboedarska rešetka
Rhombohedral (or trigonal) lattice has one lattice point at the each corner of the unit cell. It has unit cell vectors a=b=c and interaxial angles α=β=γ≠90°.
seawater → more
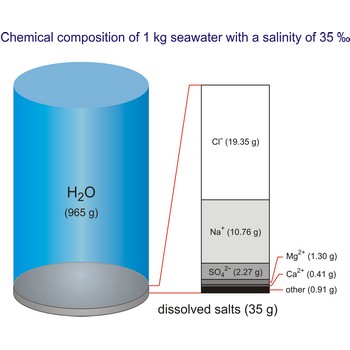
Seawater is a complex mixture of 96.5 % water, 3.5 % salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases. The world's oceans cover nearly 71 % (361 840 000 km2) of the Earth's surface (510 100 000 km2), with an average depth of 3 682.2 m.
The density of seawater is higher than that of fresh water because of its higher salinity. Seawater's freezing point is lower than that of pure water and its boiling point is higher. The average salinity of the ocean is 35 ‰, which means that for every kilograms of water, there are 35 g of salt. The relative abundance of the major salts in seawater are constant regardless of the ocean. Only six elements and compounds comprise about 99 % of sea salts: chlorine (Cl-), sodium (Na+), sulfur (SO42-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+).
significant figures → značajne znamenke
Measurements are not infinitely accurate: we must estimate measurement uncertainty. The number of significant figures is all of the certain digits plus the first uncertain digit.
Rules for significant figures:
- Disregard all initial zeros.
- Disregard all final zeros unless they follow a decimal point.
- All remaining digits including zeros between nonzero digits are significant.
| 0.0023 | has two significant figures |
| 0.109 | has three significant figures |
| 2.00 | has three significant figures |
| 70 | has one significant figure |
In addition and subtraction, the number of significant figures in the answer depends on the original number in the calculation that has the fewest digits to the right of the decimal point.
In multiplication and division, the number of significant figures in a calculated result is determined by the original measurement that has the fewest number of significant digits.
In a logarithm of a number, keep as many digits to the right of the decimal point as there are significant figures in the original number.
In an antilogarithm of a number, keep as many digits as there are digits to the right of the decimal point in the original number.
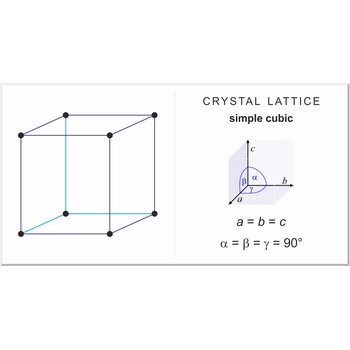
simple cubic lattice → jednostavna kubična rešetka
Simple or primitive cubic lattice (sc or cubic-P) has one lattice point at the each corner of the unit cell. It has unit cell vectors a = b = c and interaxial angels α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the sc structures the spheres fill 52 % of the volume. The number of atoms in a unit cell is one (8×1/8 = 1). This is only one metal (α-polonium) that have the sc lattice.
simple magnifier → lupa
Simple magnifier is a converging lens, placed between the object and the eye, with the object inside the focal length of the lens. The angular magnification of a simple magnifier is:
where f is the focal length of the lens and 15 cm is the near point distance for a normal eye. The image of the object is virtual, which means that the rays do not actually pass through the point of intersection, that is, it can not be seen on a screen.
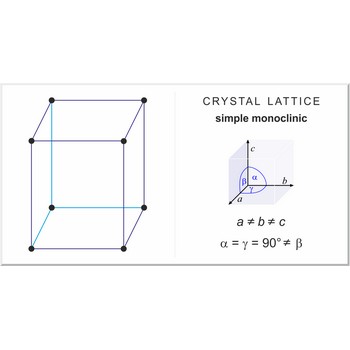
simple monoclinic lattice → jednostavna monoklinska rešetka
Simple or primitive monoclinic lattice (monoclinic-P) has one lattice point at the each corner of the unit cell. It has unit cell vectors a≠b≠c and interaxial angles α=γ=90°≠β.
simple orthorhombic lattice → jednostavna ortorompska rešetka
Simple or primitive orthorhombic lattice (orthorhombic-P) has one lattice point at the each corner of the unit cell. It has unit cell vectors a≠b≠c and interaxial angles α=β=γ=90°.
simple tetragonal lattice → jednostavna tetragonska rešetka
Simple or primitive tetragonal lattice (tetragonal-P) has one lattice point at the each corner of the unit cell. It has unit cell vectors a=b≠c and interaxial angles α=β=γ=90°.
sp3 hybrid orbital → sp3 hibridna orbitala
An sp3 hybrid orbital is an orbital formed by the linear combination of one s and three p orbitals of comparable energy (such 2s and 2p orbitals) on a same atom. The four sp3 hybrid orbitals point toward the corners of a regular tetrahedron with the bond angle of 109.5°.
state of matter → agregatno stanje
State of matter is one of the tree physical states in which matter can exist, i.e. solid, liquid or gas. Plasma is sometimes regarded as the fourth state of matter. By means of heating a solid substance will cross to liquid state at its melting point. If we heat up a liquid and beyond, at its boiling point it will cross to gaseous state - vapour.
Citing this page:
Generalic, Eni. "ON POINT." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table