period → perioda
Periods are horizontal rows in the periodic table, each period begin with an alkali metal (one electron in the outermost principal quantum level) and ending with a noble gas (each having eight electrons in the outermost principal quantum level, except for helium, which is limited to two).
periodic table of the elements → periodni sustav elemenata
Periodic table is a table of elements, written in sequence in the order of atomic number or atomic weight and arranged in horizontal rows (periods) and vertical columns (groups) to illustrate the occurrence of similarities in the properties of the elements as a periodic function of the sequence. The original form was proposed by Dmitri Mendeleev (1834-1907) in 1869, using relative atomic masses.
Petri dish → Petrijeva zdjelica
Petri dish is a shallow glass or plastic flat bottomed dish with a lid. Used primarily in laboratories for the culture of bacteria and other microorganisms on specially prepared media. It was named after the German bacteriologist Julius Richard Petri (1852-1921) who invented it in 1877.
pH → pH
pH is a convenient measure of the acid-base character of a solution, usually defined by
where c(H+) is the concentration of hydrogen ions in moles per litre. The more precise definition is in terms of activity rather than concentration.
A solution of pH 0 to 7 is acid, pH of 7 is neutral, pH over 7 to 14 is alkaline.
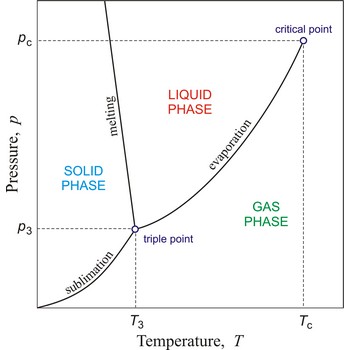
phase diagram → fazni dijagram
Phase diagram is a graphic representation of the equilibrium relationships between phases (such as vapour-liquid, liquid-solid) of a chemical compound, mixture of compounds, or solution.
The figure shows a typical phase diagram of an element or a simple compound. The stability of solid, liquid and gas phases depends on the temperature and the pressure. The three phases are in equilibrium at the triple point. The gas and liquid phases are separated by a phase transition only below the temperature of the critical point.
phenylalanine → fenilalanin
Phenylalanine is hydrophobic amino acids with aromatic side chain. It is quite hydrophobic and even the free amino acid is not very soluble in water. Phenylalanine is large aromatic residue that is normally found buried in the interior of a protein and is important for protein stability. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Phe, F
- IUPAC name: 2-amino-3-phenylpropanoic acid
- Molecular formula: C9H11NO2
- Molecular weight: 165.19 g/mol
phosphorescence → fosforescencija
Phosphorescence is emission of light from a substance exposed to radiation and persisting as an afterglow after the exciting energy has been removed. Unlike fluorescence, in which the absorbed energy is spontaneously emitted about 10-8 second after excitation, phosphorescence requires additional excitation to produce radiation and may last from about mili second to days or years, depending on the circumstances.
Citing this page:
Generalic, Eni. "OFICINAVIRTUAL.ISSSTE.GOB.MX." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table