europium → europij
Europium was discovered by Eugene Demarcay (France) in 1896. Named for the continent of Europe. It is soft, silvery-white metal. Extremely reactive with oxygen and water. Europium is obtained from monazite sand, which is a mixture of phosphates of calcium, thorium, cerium and most other rare earths. Used with yttrium oxide to make red phosphors for colour televisions.
extraction → ekstrakcija
Extraction is the separation of a component from its mixture by selective solubility. When a solution of one substance in one solvent is brought in with another solvent dissolved substance will distribute between the two solutants because of different solubility. Extraction is an efficient and fast method used for separating and concentrating matters. Extraction is best done several times in a succession, with smaller amount of solvent in it the matter is better dissolved. For example, caffeine can be separated from coffee beans by washing the beans with supercritical fluid carbon dioxide; the caffeine dissolves in the carbon dioxide, but flavour compounds do not. Vanillin can be extracted from vanilla beans by shaking the beans with an organic solvent, like ethanol.
invertase → invertaza
Invertase (sucrase, saccharase, beta-fructofuranosidase) is an enzyme present in yeast and in the intestinal juice of animals that catalyze the hydrolysis of table sugar (sucrose, saccharose) to the simple sugars, glucose and fructose. This equimolar mixture of glucose and fructose is called invert sugar.
ionic conductor → ionski vodič
Ionic conductor is a material that conducts electricity with ions as charge carriers.
ionisation energy → energija ionizacije
Ionisation energy is the minimum energy required to remove an electron from an isolated atom or molecule (in its vibrational ground state) in the gaseous phase.
ionophore → ionofore
Ionophore is a relatively small hydrophobic molecule that facilitates the transport of ions across lipid membranes. Most ionophores are produced by microorganisms. There are two types of ionophores: channel formers, which combine to form a channel in the membrane through which ions can flow; and mobile ion carriers, which transport ions across a membrane by forming a complex with the ion.
face-centered cubic lattice → plošno centrirana kubična rešetka
Face-centered cubic lattice (fcc or cubic-F), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centers of each face of the unit cell. It has unit cell vectors a =b =c and interaxial angles α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the fcc structures the spheres fill 74 % of the volume. The number of atoms in a unit cell is four (8×1/8 + 6×1/2 = 4). There are 26 metals that have the fcc lattice.
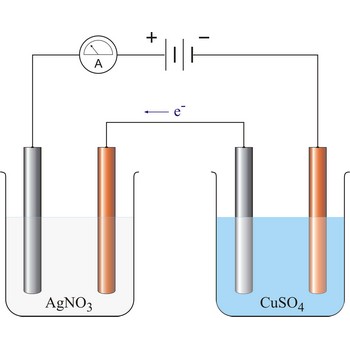
Faraday’s laws of electrolysis → Faradayevi zakoni elektrolize
Faraday’s laws of electrolysis are two laws found by British chemist and physicist Michael Faraday (1791-1867) in his experiments on electrolysis:
1. The quantity of matter extracted on the electrode is proportional to the quantity of charge (Q = I·t) which has flown in electrolysis time.
where z = number of electrons changed in reaction and F = Faraday’s constant which equals 96 487 C mol-1.
2. The masses of the elements liberated by the same quantity of electricity are directly proportional to their chemical equivalents.
96 487 C will discharge 1 mol Ag and 1/2 mol Cu. The relevant half reactions are:
Citing this page:
Generalic, Eni. "OFICINAVIRTUAL.ISSSTE.GOB.MX." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table