bronze → bronca
Bronze is an alloy made primarily of copper and tin. It may contain as much as 25 % tin. Bronzes with 10 % or more tin are harder, stronger, and resistant to corrosion. As bronze weathers, a brown or green film forms on the surface. This film inhibits corrosion. Silicon or aluminium is often added to bronze to improve resistance to corrosion. Phosphorus, lead, zinc, and other metals may be added for special purposes. The alloy is hard and easily cast and is extensively used in bearings, valves and other machine parts.
Bronze was one of the first alloys developed by ancient metal workers. The Bronze Age occurred in Europe around 2200 to 700 BC. Bronze was used for weapons such as spearheads, swords, and knives. Since ancient times, bronze has been the most popular metal for casting statues and other art objects.

The term bronze has been adopted commercially for many copper-rich alloys that contain little or no tin but are similar in colour to bronze, including aluminium bronze, manganese bronze, and silicon bronze. Aluminium bronze is used to make tools and, because it will not spark when struck. Manganese bronze is actually a brass that contains manganese. It is often used to make ship propellers because it is strong and resists corrosion by sea water.
cementation → cementacija
Cementation is any metallurgical process in which the surfaces of a metal is impregnated by some other substance, especially an obsolete process for making steel by heating bars of wrought iron to red heat for several days in a bed of charcoal.
Bunsen burner → Bunsenov plamenik
Bunsen burner is a standard source of heat in the laboratory. German chemist Roberts Bunsen (1811-1899) improved the burner's design, which had been invented by Faraday, to aid his endeavors in spectroscopy. The Bunsen burner has a vertical metal tube through which a fine jet of fuel gas is directed. Air is drawn in through airholes near the base of the tube and the mixture is ignited and burns at the tube’s upper opening. The flow of this air is controlled by an adjustable collar on the side of the metal tube. When the whole is closed a yellow safety flame is displayed. Where as when the whole is open it displays a power dull blue flame with a faint blue outer flame with a vibrant blue core used u for combustion and hearting. The flame can reach temperatures of 1 500 °C.
Bunsen, Robert Wilhem → Bunsen, Robert Wilhem
Robert Wilhem Bunsen (1811-1899) is a German chemist who held professorships at Kassel, Marburg and Heidelberg. His early researches on organometallic compound of arsenic cost him an eye in an explosion. Bunsen's most important work was in developing several techniques used in separating, identifying, and measuring various chemical substances. He also improvement chemical battery for use in isolating quantities of pure metals - Bunsen battery.
The essential piece of laboratory equipment that has immortalized the name of Bunsen was not invented by him. Bunsen improved the burner's design, which had been invented by Faraday, to aid his endeavors in spectroscopy. Use of the Bunsen burner in conjunction with a glass prism led to the development of the spectroscope in collaboration with the German physicist Gustav Kirchoff and to the spectroscopic discovery of the elements rubidium (1860) and cesium (1861).
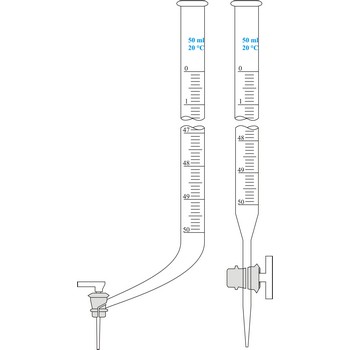
burette → bireta
Burette is a graded glass pipe which on its lower side has a glass faucet by which it can drop a precise quantity of liquid. Inner diameter of a burette must be equal in its whole length, because the accuracy of volume measurement depends upon that. Burettes are primarily used in volumetric analysis for titration with standard solution reagent. Most often Schellbach’s burette is used, graded on 50 mL with division of scale on 0.1 mL. Every burette is calibrated on discharge. For serial determining automatic burettes are used.
centrifugal force → centrifugalna sila
Centrifugal force is a force which moves a body away from the centre of motion.
centripetal force → centripetalna sila
Centripetal force is a force which makes body move through a curvature. Its orientation is always in path forwards the centre of rotation.
Citing this page:
Generalic, Eni. "OFICINAVIRTUAL.ISSSTE.GOB.MX." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table