IUPAC → IUPAC
The International Union of Pure and Applied Chemistry (IUPAC) is a non-governmental organization of member countries that encompasses more than 85% of the world’s chemical sciences and industries. It was formed in 1919 with the object of facilitating international agreement and uniform practice in both academic and industrial aspects of chemistry.
absorptance → apsorptancija
Absorptance (α) is the ratio of the radiant or luminous flux in a given spectral interval absorbed in a medium to that of the incident radiation. Also called absorption factor.
acid → kiselina
Acid is a type of compound that contains hydrogen and dissociates in water to produce positive hydrogen ions. The reaction for an acid HA is commonly written:
In fact, the hydrogen ion (the proton) is solvated, and the complete reaction is:
This definition of acids comes from the Arrhenius theory. Such acids tend to be corrosive substances with a sharp taste, which turn litmus red and produce colour changes with other indicators. They are referred to as protonic acids and are classified into strong acids, which are almost completely dissociated in water, (e.g. sulphuric acid and hydrochloric acid), and weak acids, which are only partially dissociated (e.g. acetic acid and hydrogen sulphide). The strength of an acid depends on the extent to which it dissociates, and is measured by its dissociation constant.
In the Lowry-Brønsted theory of acids and bases (1923), the definition was extended to one in which an acid is a proton donor (a Brønsted acid), and a base is a proton acceptor (a Brønsted base). An important feature of the Lowry-Brønsted concept is that when an acid gives up a proton, a conjugate base is formed that is capable of accepting a proton.
Similarly, every base produces its conjugate acid as a result of accepting a proton.
For example, acetate ion is the conjugate base of acetic acid, and ammonium ion is the conjugate acid of ammonia.
As the acid of a conjugate acid/base pair becomes weaker, its conjugate base becomes stronger and vice versa.
A further extension of the idea of acids and bases was made in the Lewis theory. In this, a G. N. Lewis acid is a compound or atom that can accept a pair of electrons and a Lewis base is one that can donate an electron pair. This definition encompasses "traditional" acid-base reactions, but it also includes reactions that do not involve ions, e.g.
in which NH3 is the base (donor) and BCl3 the acid (acceptor).
alkalinity → alkalitet
Alkalinity is a measure of a material’s ability to neutralize acids. It is usually determined using titration.
Bravais lattice → Bravaisova rešetka
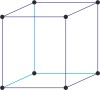
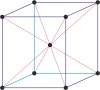
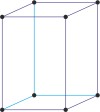
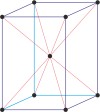
Bravais lattice is a set of points constructed by translating a single point in discrete steps by a set of basis vectors. The French crystallographer Auguste Bravais (1811-1863) established that in three-dimensional space only fourteen different lattices may be constructed. All crystalline materials recognised till now fit in one of these arrangements. The fourteen three-dimensional lattices, classified by crystal system, are shown to the bottom.
|
Crystal system
|
Bravais lattices
|
|||
|
cubic a=b=c α=β=γ=90° |
 |
 |
 |
|
|
|
simple cubic
|
body-centered cubic
|
face-centered cubic
|
|
|
tetragonal a=b≠c α=β=γ=90° |
 |
 |
||
|
|
simple tetragonal
|
body-centered tetragonal
|
||
|
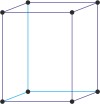
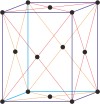
orthorhombic a≠b≠c α=β=γ=90° |
 |
 |
 |
 |
|
|
simple orthorhombic
|
base-centered orthorhombic
|
body-centered orthorhombic
|
face-centered orthorhombic
|
|
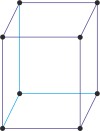
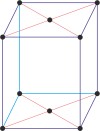
monoclinic a≠b≠c α=γ=90°≠β |
 |
 |
||
|
|
simple monoclinic
|
base-centered monoclinic
|
||
|
hexagonal a=b≠c α=β=90° γ=120° |
 |
|||
|
|
hexagonal
|
|||
|
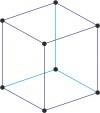
rhombohedral a=b=c α=β=γ≠90° |
 |
|||
|
|
rhombohedral
|
|||
|
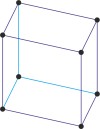
triclinic a≠b≠c α≠β≠γ≠90° |
 |
|||
|
triclinic
|
||||
butane → butan
Butane is a gaseous hydrocarbon C4H10 obtained from petroleum (refinery gas or by cracking higher hydrocarbons). The fourth member of the alkane series, it has a straight chain of carbon atoms and is isomeric with 2-methylpropane, formerly called isobutene. It can easily be liquefied under pressure and is supplied into cylinders for use as a fuel gas. It is also a raw material for making buta-1, 3-diene for synthetic rubber.
acid-base titration → kiselo-bazna titracija
Acid-base titration is an analytical technique in volumetric analysis, where an acid of known concentration is used to neutralise a known volume of a base, and the observed volume of the acid required is used to determine the unknown concentration of the base. An acid-base indicator is used to determine the end-point of the titration.
alkanes → alkani
Alkanes (paraffins) are acyclic branched or unbranched hydrocarbons having the general formula CnH2n+2, and therefore consisting entirely of hydrogen atoms and saturated carbon atoms. In the systematic chemical nomenclature alkane names end in the suffix -ane. They form a homologous series (the alkane series) methane (CH4), ethane (C2H6), propane (C3H8), butane (C4H10), etc. The lower members of the series are gases; the high-molecular mass alkanes are waxy solid. Generaly the alkanes are fairly unreactive. They form haloalkanes with halogens when irradiated with ultraviolet radiation. Alkanes are present in natural gas and petroleum.
Citing this page:
Generalic, Eni. "Neutral face meme." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table


