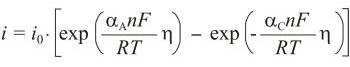Nernst’s electrode potential equation → Nernstova jednadžba za elektrodni potencijal
For general reaction of some redox system
dependence of electrode potential of redox system upon activity of oxidised and reduced form in solution is described in Nernst’s equation for electrode potential:
where E = to electrode potential of redox system
E° = standard electrode potential of redox system
R = universal gas constant
T = thermodymical temperature
F = Faraday’s constant
z = number of electrons exchanged in redox reaction
aO = activity of oxidised form
aR = activity of reduced form
n = stechiometrical coefficient of oxidised form
m = stechiometrical coefficient of reduced form
conditional electrode potential → uvjetni elektrodni potencijal
Conditional or formal electrode potential (E°’) is equal to electrode potential (E) when overall concentrations of oxidised and reduced form in all its forms in a solution are equal to one. Conditional electrode potential includes all effects made by reactions that do not take part in the electron exchange, but lead to change of ion power, changes of pH, hydrolysis, complexing, precipitating, etc.
At 298 K (25 °C) and by converting natural (Napierian) logarithms into decimal (common, or Briggian) logarithms, Nernst’s equation for electrode potential can be written as follows:
standard electrode potential → standardni elektrodni potencijal
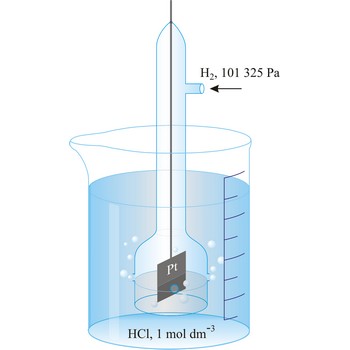
Standard electrode potential (E°) (standard reduction potentials) are defined by measuring the potential relative to a standard hydrogen electrode using 1 mol solution at 25 °C. The convention is to designate the cell so that the oxidised form is written first. For example,
The e.m.f. of this cell is -0.76 V and the standard electrode potential of the Zn2+|Zn half cell is -0.76 V.
electrode potential → elektrodni potencijal
Electrode potential is defined as the potential of a cell consisting of the electrode in question acting as a cathode and the standard hydrogen electrode acting as an anode. Reduction always takes place at the cathode, and oxidation at the anode. According to the IUPAC convention, the term electrode potential is reserved exclusively to describe half-reactions written as reductions. The sign of the half-cell in question determines the sign of an electrode potential when it is coupled to a standard hydrogen electrode.
Electrode potential is defined by measuring the potential relative to a standard hydrogen half cell
The convention is to designate the cell so that the oxidised form is written first. For example
The e.m.f. of this cell is
By convention, at p(H2) = 101325 Pa and a(H+) = 1.00, the potential of the standard hydrogen electrode is 0.000 V at all temperatures. As a consequence of this definition, any potential developed in a galvanic cell consisting of a standard hydrogen electrode and some other electrode is attributed entirely to the other electrode
Heyrovsky-Ilkovic equation → Heyrovsky-Ilkovičeva jednadžba
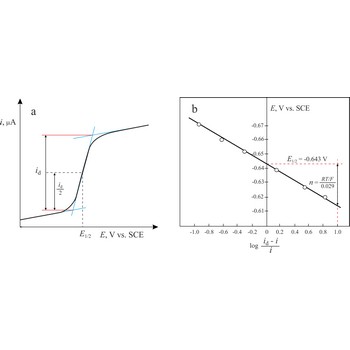
The Heyrovsky-Ilkovic equation describes the entire current-potential curve (polarographic wave) of a reversible redox system in polarography
where R is the gas constant, T is the absolute temperature, F is the Faraday constant, n denotes the number of electrons taking part in the electrode reaction. E1/2 is a unique potential (for a given reaction and supporting electrolyte) termed the half-wave potential.
In order to obtain E1/2 from the above equation, we plot a graph of ln[(id-i)/i] against E. The intercept on the x-axis gives then an accurate value of E1/2. The slope of the obtained straight line is equal to nF/RT from which n is determined.
Butler-Volmer equation → Butler-Volmerova jednadžba
Butler-Volmer equation is an activation controlled reaction, the one for which the rate of reaction is controlled solely by the rate of the electrochemical charge transfer process, which is in turn an activation-controlled process. This gives rise to kinetics that are described by the Butler-Volmer equation:
where io is exchange current density, η is overpotential (η = E - Eo), n is number of electrons, αA is anodic transfer coefficient, and αC is cathodic transfer coefficient
cell potential → potencijal članka
Cell potential (E) is difference between anode and cathode potential. If the cell potential is positive, then the reaction is spontaneous.
redox potential → redoks potencijal
Redox potential is the potential of a reversible oxidation-reduction electrode measured with respect to a reference electrode, corrected to the hydrogen electrode, in a given electrolyte.
Schrodinger equation → Schrodingerova jednadžba
Schrödinger equation is the basic equation of wave mechanics which, for systems not dependent on time, takes the form:
where Ψ is the wavefunction, V is the potential energy expressed as a function of the spatial coordinates, E its total energy, ![]() 2 is the Laplacian operator, h is Planck’s constant, and m is the mass.
2 is the Laplacian operator, h is Planck’s constant, and m is the mass.
Arrhenius equation → Arheniusova jednadžba
In 1889, Svante Arrhenius explained the variation of rate constants with temperature for several elementary reactions using the relationship
where the rate constant k is the total frequency of collisions between reaction molecules A times the fraction of collisions exp(-Ea/RT) that have an energy that exceeds a threshold activation energy Ea at a temperature of T (in kelvin). R is the universal gas constant.
Citing this page:
Generalic, Eni. "Nernstova jednadžba za elektrodni potencijal." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table