fat → mast
Fats are esters of glycerol and long chain carboxylic acids. Fats occur widely in plants and animals as a means of storing food energy, having twice the calorific value of carbohydrates. Fats derived from plants and fish generally have a greater proportion of unsaturated fatty acids than those from mammals. Fats may be either solid or liquid at room temperature, depending on their structure and composition. Unsaturated fats are liquid at room temperature.
Plant oils may be hardened by the addition of hydrogen atoms, converting double bonds to single bonds. This process is known as hydrogenation. Hydrogenated vegetable oils are often present in margarine and other processed foods.
Alkali hydrolysis of fat with sodium hydroxide it gives glycerol and soap (i.e. a mixture of the sodium salts of the fatty acids).
oligomer → oligomer
Oligomer is a substance consisting of molecules of intermediate relative molecular mass (molecular weight), the structure of which essentially comprises the multiple repetitions of units derived, actually or conceptually, from molecules of low relative molecular mass. In contrast to a polymer, the properties of an oligomer can vary significantly with the removal of one or a few of its units.
fructose → fruktoza
Fructose (fruit sugar) is a ketohexose (a six-carbon ketonic sugar), which occurs in sweet fruits and honey. Glucose and fructose have the same molecular formula, C6H12O6, but have different structures. Pure, dry fructose is a very sweet, white, odorless, crystalline solid. Fructose is one of the sweetest of all sugars and is combined with glucose to make sucrose, or common table sugar. An older common name for fructose is levulose, after its levorotatory property of rotating plane polarized light to the left (in contrast to glucose which is dextrorotatory). The polysaccharide inulin is a polymer of fructose.
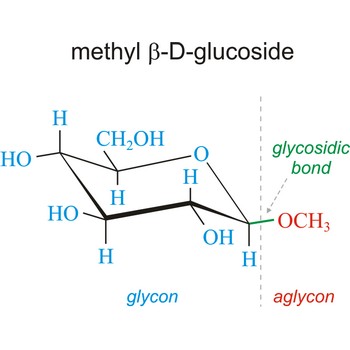
glycosidic bond → glikozidna veza
Glycosidic bond ia a bond between the glycosyl group, the structure obtained by removing the hydroxy group from the hemiacetal function of a monosaccharide, and the -OR group (which itself may be derived from a saccharide and chalcogen replacements thereof (RS–, RSe–). The terms N-glycosides and C-glycosides are misnomers and should not be used. The glycosidic bond can be α or β in orientation, depending on whether the anomeric hydroxyl group was α or β before the glycosidic bond was formed and on the specificity of the enzymatic reaction catalyzing their formation. Once the glycosidic bond is formed, the anomeric configuration of the ring is locked as either α or β. Specific glycosidic bonds therefore may be designated α(1→4), β(1→4), α(1→6), and so on. Cellulose is formed of glucose molecules linked by β(1→4)-glycosidic bonds, whereas starch is composed of α(1→4)-glycosidic bonds.
percentage composition of atom in the molecule → postotni sastav atoma u molekuli
Percentage composition of atom in the molecule is a structure of compound presented in the shape of a percentage of its mass, which comes from every element.
Citing this page:
Generalic, Eni. "Lewis structure." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table