sedimentary rocks → sedimentne stijene
Sedimentary Rocks are formed by the accumulation and subsequent consolidation of sediments into various types of rock. There are three major types of sedimentary rocks:
Biogenic sedimentary rocks are formed from organic processes when organisms use materials dissolved in water to build a shell or other skeletal structure.
Clastic sedimentary rocks are composed directly of the sediments or fragments from other rocks.
Chemical sedimentary rocks are formed through evaporation of a chemical rich solution.
Based on their sizes, sediment particles are classified, based on their size, into six general categories:
- boulder (>256 mm)
- cobble (64 - 256 mm)
- gravel (2 - 64 mm)
- sand (1/16 - 2 mm)
- silt (1/256 - 1/16 mm)
- clay (<1/256 mm)
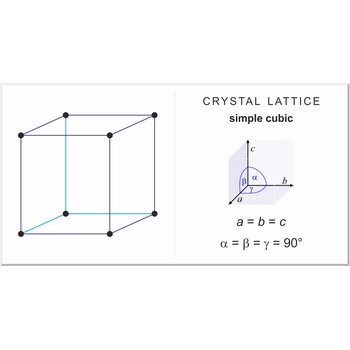
simple cubic lattice → jednostavna kubična rešetka
Simple or primitive cubic lattice (sc or cubic-P) has one lattice point at the each corner of the unit cell. It has unit cell vectors a = b = c and interaxial angels α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the sc structures the spheres fill 52 % of the volume. The number of atoms in a unit cell is one (8×1/8 = 1). This is only one metal (α-polonium) that have the sc lattice.
solar cell → sunčeva ćelija
Solar cell, or photovoltaic cell, is a device that captures sunlight and transforms it directly to electricity. All solar cells make use of photovoltaic effect, so often they are called photovoltaic cells. Almost all solar cells are built from solid-state semiconducting materials, and in the vast majority of these the semiconductor is silicon.
The photovoltaic effect involves the generation of mobile charge carriers-electrons and positively charged holes-by the absorption of a photon of light. This pair of charge carriers is produced when an electron in the highest filled electronic band of a semiconductor (the valence band) absorbs a photon of sufficient energy to promote it into the empty energy band (the conduction band). The excitation process can be induced only by a photon with an energy corresponding to the width of the energy gap that separates the valence and the conduction band. The creation of an electron-hole pair can be converted into the generation of an electrical current in a semiconductor junction device, wherein a layer of semiconducting material lies back to back with a layer of either a different semiconductor or a metal. In most photovoltaic cells, the junction is p-n junction, in which p-doped and n-doped semiconductors are married together. At the interface of the two, the predominance of positively charged carriers (holes) in the p-doped material and of negatively charged carriers (electrons) in the n-doped material sets up an electric field, which falls off to either side of the junction across a space-charge region. When absorption of a photon in this region generates an electron-hole pair, these charge carriers are driven in opposite directions by the electric field, i.e. away from the interface and toward the top and bottom of the two-layer structure, where metal electrodes on these faces collect the current. The electrode on the top layer (through which light is absorbed) is divided into strips so as not to obscure the semiconducting layers below. In most widely used commercial solar cells, the p-doped and n-doped semiconductive layers are formed within a monolithic piece of crystalline silicon. Silicon is able to absorb sunlight at those wavelengths at which it is most intense-from the near-infrared region (wavelengths of around 1200 nm) to the violet (around 350 nm).
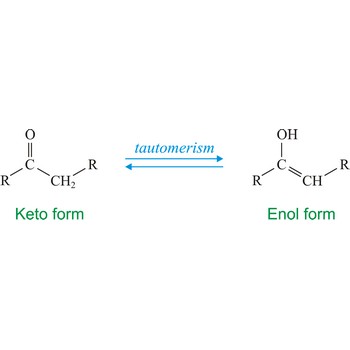
tautomerism → tautomerija
Tautomerism refers to equilibrium between two different structures of the same compound. Usually the tautomers differ in the point of attachment of a hydrogen atom. One of the most common examples of a tautomeric system is the equilibrium between a ketone (keto) and aldehyde (enol).
thermosetting plastic → termostabilna plastika
Thermosetting plastics (thermosets) refer to a range of polymer materials that cure, through the addition of energy, to a stronger form. The energy may be in the form of heat (rubber), through a chemical reaction (two part epoxy), or irradiation. Thermoset materials are usually liquid or malleable prior to curing, and designed to be molded into their final form, or used as adhesive.
Thermoset polymer resins transformed into plastics or rubbers by cross-linking into a rigid, 3-D structure. A thermoset material cannot be melted and re-molded after it is cured.
transition metal → prijelazni element
This group of metals is distinguished from other metals not by their physical properties, but by their electronic structure. Transition metals are elements characterized by a partially filled d subshell. The First Transition Series comprises scandium (Sc), titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni) and copper (Cu). The Second and Third Transition Series include the lanthanides and actinides, respectively.
The transition metals are noted for their variability in oxidation state. Thus, manganese has two electrons in its outside shell and five electrons in the next shell down, and exhibits oxidation states of +1, +2, +3, +4, +5, +6, and +7.
They are also characterised by the fact that well into the series, going from left to right, the properties of the succeeding metals do not differ greatly from the preceding ones.
valine → valin
Valine is hydrophobic amino acids with aliphatic side chain. It is a member of the branched-chain amino acid family, along with leucine and isoleucine. Valine differs from threonine by replacement of the hydroxyl group with a methyl substituent, but they are of roughly the same shape and volume. The nonpolar hydrophobic amino acids tend to cluster together within proteins, stabilizing protein structure by means of hydrophobic interactions. Valine is an essential amino acid, which means that it cannot be synthesized in the body and must be obtained through dietary sources.
- Abbreviations: Val, V
- IUPAC name: 2-amino-3-methylbutanoic acid
- Molecular formula: C5H11NO2
- Molecular weight: 117.15 g/mol
X-rays → X-zrake
X-rays are electromagnetic radiation of shorter wavelength than ultraviolet radiation (10-11 m to 10-9 m or 0.01 nm to 1 nm) produced by bombardment of atoms by high-quantum-energy particles. X-rays can pass through many forms of matter and they are therefore used medically and industrially to examine the internal structure.

zeolite → zeolit
Zeolite is a natural or synthetic hydrated aluminosilicate with an open three-dimensional crystal structure, in which water molecules are held in cavites in the latice. The water can be driven off by heating and the zeolite can then absorb other molecules of suitable size. Zeolites are used for separating mixtures by selective absorption.
Chitin → Hitin
Chitin is a nitrogen-containing linear polysaccharide of ß(1->4) linked units of N-acetyl-ß-d-glucosamine. The structure of chitin is similar to cellulose except for the replacement hydroxyl group (-OH) at the carbon 2 with an acetyl amine group (–NH–CO–CH3). Chitin is the main component of the exoskeleton, or outer covering of insects, crustaceans, and arachnids. It is also found in the cell walls of certain fungi and algae. After cellulose, chitin is the second most abundant biopolymer in nature. It is insoluble in water, organic solvents, weak acids and lyes.
Citing this page:
Generalic, Eni. "Lewis structure." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table