valence electron → valentni elektron
Valence electrons are electrons that can be actively involved in a chemical change, usually electrons in the outermost (valent) shell. For example, sodium’s ground state electron configuration is 1s2 2s2 2p6 3s1; the 3s electron is the only valence electron in the atom. Germanium (Ge) has the ground state electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2; the 4s and 4p electrons are the valence electrons.
work → rad
Work is the energy required to move an object against an opposing force. Work is usually expressed as a force times a displacement.
When a constant force F acts on a point-like object while the object moves through a displacement s, the force does work W on the object. If force and displacement are at a constant angle Θ to each other, the work is expressed by the scalar product of these two vectors:
When the force F on a point-like object is not constant that is, it depends on the position of the object, the work done by force while object moves from initial position with coordinates (xi, yi, zi) to final position with coordinates (xf, yf, zf)is given by expression:
Where Fx, Fy and Fz are scalar components of the force.
SI unit for work is joule (J); 1 J = 1 Nm = 1 kg m2 s-2. The electron-volt (eV) is commonly used in atomic and nuclear physics.
X-ray diffraction → rendgenska difrakcija
The regular array of atoms in a crystal is a three-dimensional diffraction grating for short-wavelength waves such as X-rays. The atoms are arranged in planes with interplanar spacing d. Diffraction maxima occur in the incident direction of the wave, measured from the surface of a plane of atoms, and the wavelength λ of the radiation satisfy Braggs’s law:
X-rays → X-zrake
X-rays are electromagnetic radiation of shorter wavelength than ultraviolet radiation (10-11 m to 10-9 m or 0.01 nm to 1 nm) produced by bombardment of atoms by high-quantum-energy particles. X-rays can pass through many forms of matter and they are therefore used medically and industrially to examine the internal structure.

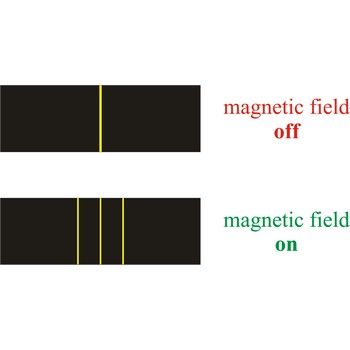
Zeeman effect → Zeemanov efekt
Zeeman effect is the splitting of the lines in a spectrum when the source of the spectrum is exposed to a magnetic field. The effect was discovered in 1896 by the Dutch physicist Pieter Zeeman (1865-1943) as a broadening of the yellow D-lines of sodium in a flame held between strong magnetic poles.
The Zeeman effect has helped physicists determine the energy levels in atoms. In astronomy, the Zeeman effect is used in measuring the magnetic field of the Sun and of other stars.
Lennard-Jones potential → Lennard-Jonesov potencijal
The Lennard-Jones potential (or 12-6 potential) is a mathematically simple model that describes the interaction between two non-bonded and uncharged atoms (known as the van der Waals interaction). It was first proposed in 1924 by British physicist Sir John Edward Lennard-Jones (1894-1954). The Lennard-Jones Potential is given by the following equation
V(r) = 4e[(sigma/r)12-(sigma/r)6)]
where V is the intermolecular potential between the two atoms or molecules, ε is the well depth and a measure of how strongly the two particles attract each other, σ is the distance at which the intermolecular potential between the two particles is zero, r is the distance of separation between centres of both particles.
Citing this page:
Generalic, Eni. "Chaotic Atoms (Dramatic)." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table