structural formula → strukturna formula
Structural formula is a two dimensional representations of the arrangement of the atoms in molecules. Atoms are represented by their element symbols and covalent bonds are represented by lines. The symbol for carbon is often not drawn.
T-shaped molecular geometry → T-oblik geometrije molekule
T-shape is a molecular geometry that results when there are 3 bonds and 2 lone pairs around the central atom in the molecule. The atoms bonded to the central atom lie at the ends of a T with 90° angles between them. Molecules with an trigonal bipyramidal electron pair geometries have sp3d (or dsp3) hybridization at the central atom. ICl3 has a T-shaped molecular geometry.
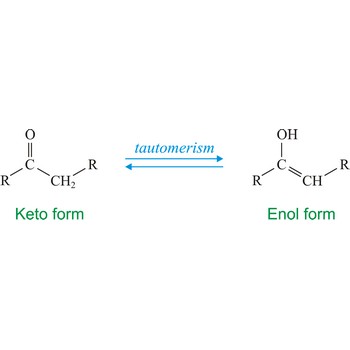
tautomerism → tautomerija
Tautomerism refers to equilibrium between two different structures of the same compound. Usually the tautomers differ in the point of attachment of a hydrogen atom. One of the most common examples of a tautomeric system is the equilibrium between a ketone (keto) and aldehyde (enol).
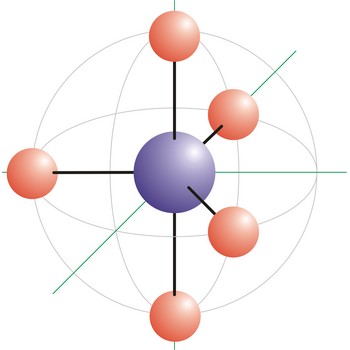
tetrahedral molecular geometry → tetraedarska geometrija molekule
Tetrahedral is a molecular shape that results when there are four bonds and no lone pairs around the central atom in the molecule. The atoms bonded to the central atom lie at the corners of a tetrahedron with 109.5° angles between them. Molecules with an tetrahedral electron pair geometries have sp3 hybridization at the central atom. The ammonium ion (NH4+) and methane (CH4) have a tetrahedral molecular geometry.
trigonal bipyramidal molecular geometry → trigonska bipiramidalna geometrija molekule
Trigonal bipyramidal (trigonal bipyramidal shape) is a molecular geometry that results when there are five bonds and no lone pairs on the central atom in the molecule. Three of the bonds are arranged along the atom’s equator, with 120° angles between them; the other two are placed at the atom’s axis. Axial bonds are at right angles to the equatorial bonds. Molecules with an trigonal bipyramidal electron pair geometries have sp3d (or dsp3) hybridization at the central atom. The PCl5 molecule has a trigonal bipyramidal molecular geometry.
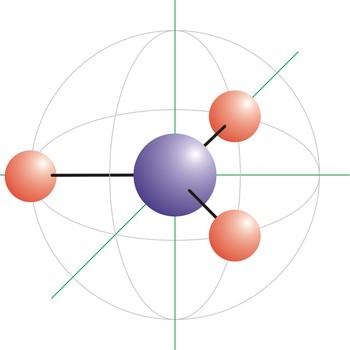
trigonal planar molecular geometry → trigonska planarna geometrija molekule
Trigonal planar is a molecular shape that results when there are three bonds and no lone pairs around the central atom in the molecule. The pairs are arranged along the central atom’s equator, with 120° angles between them. Molecules with an trigonal planar electron pair geometries have sp2d hybridization at the central atom. The carbonate ion (CO32-) has a trigonal planar geometry.
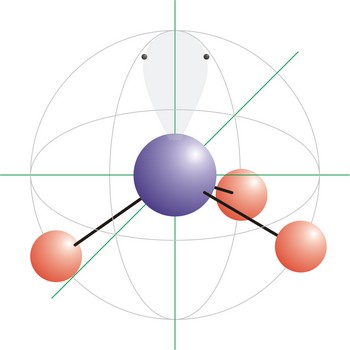
trigonal pyramidal molecular geometry → trigonska piramidalna geometrija molekule
Trigonal pyramidal is a molecular shape that results when there are three bonds and one lone pair on the central atom in the molecule. Molecules with an tetrahedral electron pair geometries have sp3 hybridization at the central atom. Ammonia (NH3) is a trigonal pyramidal molecule.
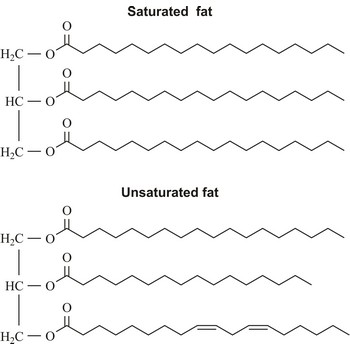
tryglyceride → triglicerid
Triglyceride is an ester of glycerol and three fatty acids. It is the main constituent of vegetable oil and animal fats. The fatty acids attached to the glycerol can be the same or different. Natural fatty acids found in plants and animals are typically composed only of even numbers of carbon atoms (usually from 16 to 20) due to the way they are bio-synthesized from acetyl CoA.
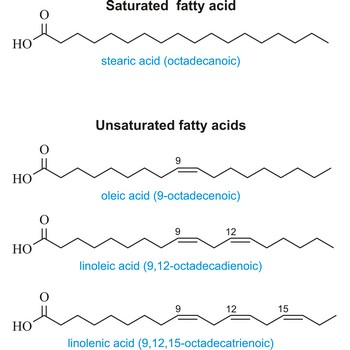
unsaturated fatty acid → nezasićena masna kiselina
Unsaturated fatty acid is a fatty acid whose carbon chain can absorb additional hydrogen atoms. Their carbon chain has one or more double or triple valence bond per molecule. The most important of these are:
| Oleic (9-octadecenoic acid) | CH3(CH2)7CH=CH(CH2)7COOH |
| Linoleic (9,12-octadecadienoic acid) | CH3(CHCH2)3(CH2CH=CH)2(CHCH2)7COOH |
| Linolenic (9,12,15-octadecatrienoic acid) | CH3(CH2CH=CH)3(CHCH2)7COOH |
ununquadium → ununkvadij
The discovery of ununquadium was reported informally in January 1999 following experiments towards the end of December 1998 involving scientists at Dubna (Joint Institute for Nuclear Research) in Russia and the Lawrence Livermore National Laboratory, USA. The new element has not yet been officially named, but it is known as ununquadium, according to the system designated by the IUPAC for naming new elements. It is synthetic radioactive metal. Only few atoms of element 114 (289114) has ever been made (through a nuclear reaction involving fusing a calcium atom with a plutonium atom) isolation of an observable quantity has never been achieved.
Citing this page:
Generalic, Eni. "Chaotic Atoms (Dramatic)." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table