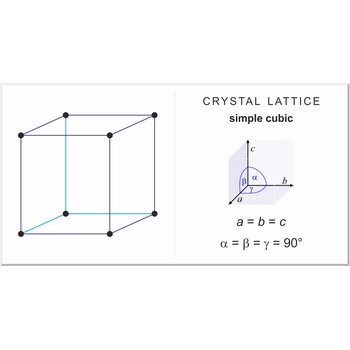
simple cubic lattice → jednostavna kubična rešetka
Simple or primitive cubic lattice (sc or cubic-P) has one lattice point at the each corner of the unit cell. It has unit cell vectors a = b = c and interaxial angels α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the sc structures the spheres fill 52 % of the volume. The number of atoms in a unit cell is one (8×1/8 = 1). This is only one metal (α-polonium) that have the sc lattice.
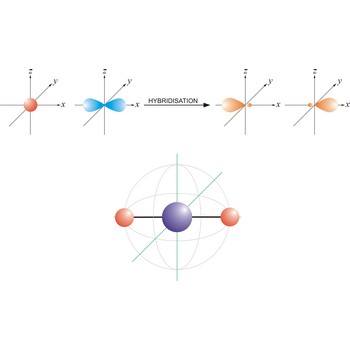
sp hybrid orbital → sp hibridna orbitala
An sp hybrid orbital is an orbital formed by the linear combination of one s and one p orbital of comparable energy (such 2s and 2p orbitals) on a same atom. The two sp hybrid orbitals are aligned in a straight line in opposite direction (bond angles are 180°). The remaining two p orbitals are at right angles to one another and to the line formed by the two sp orbitals.
sp2 hybrid orbital → sp2 hibridna orbitala
An sp2 hybrid orbital is an orbital formed by the linear combination of one s and two p orbitals of comparable energy (such 2s and 2p orbitals) on a same atom. The three sp2 hybrid orbitals lie in a plane with angle of 120°. The remaining p orbital remains unchanged and is perpendicular to the plane of the three sp2 orbitals.
sp3 hybrid orbital → sp3 hibridna orbitala
An sp3 hybrid orbital is an orbital formed by the linear combination of one s and three p orbitals of comparable energy (such 2s and 2p orbitals) on a same atom. The four sp3 hybrid orbitals point toward the corners of a regular tetrahedron with the bond angle of 109.5°.
spin → spin
Spin is the intrinsic angular momentum of an elementary particle, or system of particles such as nucleus, that is also responsible for the magnetic moment; or, a particle or nucleus possessing such a spin. The spins of nuclei have characteristic fixed values. Pairs of neutrons and protons align to cancel out their spins, so that nuclei with an odd number of neutrons and/or protons will have a net non-zero rotational component characterized by a non-zero quantum nuclear spin number.
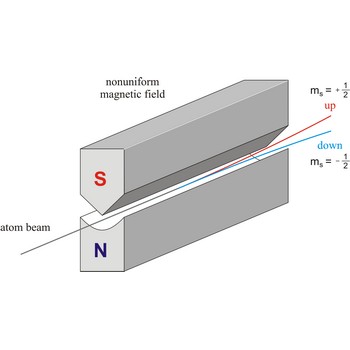
Stern-Gerlach experiment: a beam of silver atoms is split into two beams when it traverses a nonuniform magnetic field. Atoms with spin quantum number ms=+1/2 follow one trajectory, and those with ms=+1/2 follow another.
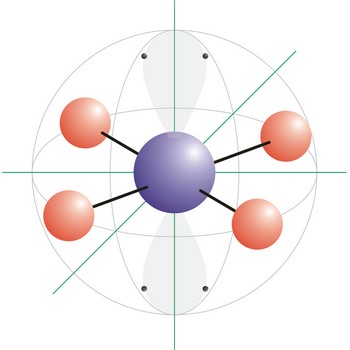
square planar molecular geometry → kvadratna planarna geometrija molekule
Square planar is a molecular shape that results when there are four bonds and two lone pairs on the central atom in the molecule. An example of a square planar molecule is xenon tetrafluoride (XeF4). This molecule is made up of six equally spaced sp3d2 (or d2sp3) hybrid orbitals arranged at 90° angles. The shape of the orbitals is octahedral. Two orbitals contain lone pairs of electrons on opposite sides of the central atom. The remaining four atoms connected to the central atom give the molecule a square planar shape.
square pyramidal molecular geometry → kvadratna piramidalna geometrija molekule
Square pyramidal is a molecular shape that results when there are five bonds and one lone pair on the central atom in the molecule. Bromine pentafluoride (BrF5) has the geometry of a square pyramid, with fluorine atoms occupying five vertices, one of which is above the plane of the other four. This molecule is made up of six equally spaced sp3d2 (or d2sp3) hybrid orbitals arranged at 90° angles. The shape of the orbitals is octahedral. Because of the high symmetry of the octahedral arrangement, all six positions are equivalent, so it does not matter in which position in the drawing we put the lone pair. The remaining four atoms connected to the central atom give the molecule a square planar shape.
stereoisomer → stereoizomer
Stereoisomers are compounds that have identical chemical constitution, but differ as regards the arrangement of the atoms or groups in space. Stereoisomers fall into two broad classes: optical isomers (enantiomers) and geometric isomers (cis-trans).
strontium → stroncij
Strontium was discovered by Sir Humphry Davy (England) in 1808. Named after the village of Strontian in Scotland. It is soft, malleable, silvery-yellow metal. Combustible in air, will react with water. Exposed surfaces form protective oxide film. Metal ignites and burns readily. Strontium is found in minerals celestite and strontianite. Used in flares and fireworks for crimson colour. Strontium-90 is a long lived highly radioactive fallout product of atomic-bomb explosions.
Citing this page:
Generalic, Eni. "Chaotic Atoms (Dramatic)." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table