primary alcohol → primarni alkohol
Primary alcohols are alcohols where the hydroxyl group is attached to a primary carbon atom. Thus, it has the general structure, RCH2OH, where R is a hydrogen atom or an alkyl group.
radioactivity → radioaktivnost
Radioactivity is capability of a spontaneous decay of an atom. In this way a new atom type is formed and radioactive radiation is released. An atom can emit three types of radioactive radiation: positive α-radiation, negative β-radiation and electrically neutral γ-radiation. During radioactive decay one element never emits all types of radiation at the same time.
gamma radiation → gama-zračenje
Gamma radiation is electromagnetic radiation of extremely short wavelength. Gamma radiation ranges in energy from about 10-15 J to 10-10 J (10 keV to 10 MeV) (wavelength less than about 1 pm). Gamma rays are emitted by excited atomic nuclei during the process of passing to a lower excitation state.
Gamma rays are extremely penetrating and are absorbed by dense materials like lead and uranium. Exposure to gamma radiation may be lethal.
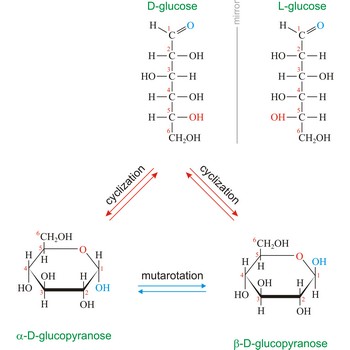
glucose → glukoza
Glucose (grape sugar, blood sugar), C6H12O6, is an aldohexose (a monosaccharide sugar having six carbon atoms and an aldehyde group). An older common name for glucose is dextrose, after its dextrorotatory property of rotating plane polarized light to the right. Glucose in free (in sweet fruits and honey) or combined form (sucrose, starch, cellulose, glycogen) is is probably the most abundant organic compound in nature. During the photosynthesis process, plants use energy from the sun, water from the soil and carbon dioxide gas from the air to make glucose. In cellular respiration, glucose is ultimately broken down to yield carbon dioxide and water, and the energy from this process is stored as ATP molecules (36 molecules of ATP across all processes).
Naturally occurring glucose is D isomers (OH group on the stereogenic carbon farthest from the aldehyde group, C-5, is to the right in the Fischer projection). Although often displayed as an open chain structure, glucose and most common sugars exist as ring structures. In the α form, the hydroxyl group attached to C-1 and the CH2OH attached to C-5 are located on opposite sides of the ring. β-glucose has these two groups on the same side of the ring. The full names for these two anomers of glucose are α-D-glucopyranose and β-D-glucopyranose.
secondary alcohol → sekundarni alkohol
Secondary alcohol is one in which the hydroxyl group (-OH) is attached to a secondary carbon atom (i.e. a carbon atom which has one hydrogen atom attached to it).
solid solution → čvrste otopine
Solid solution is a crystalline material that is a mixture of two or more components, with ions, atoms, or molecules of one component replacing some of the ions, atoms of the other component in its normal crystal lattice.
graphite → grafit
Graphite is an allotrope of carbon. The atoms are arranged in layers as a series of flat, hexagonal rings. Graphite is a good conductor of heat and electricity. The layers cleave easily, making graphite useful as a solid lubricant. A process to make pure synthetic graphite was invented by the American chemist Edward Goodrich Acheson (1856–1931). The process consists of heating a mixture of clay (aluminum silicate) and powdered coke (carbon) in an iron bowl. The reaction involves the production of silicon carbide, which loses silicon at 4150 °C to leave graphite.
Citing this page:
Generalic, Eni. "Chaotic Atoms (Dramatic)." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table