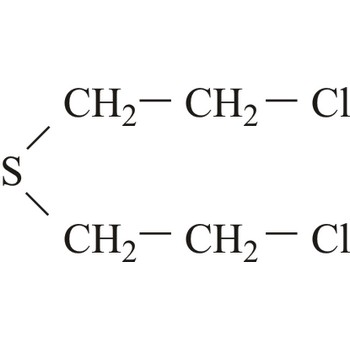glycine → glicin
Glycine is the smallest amino acid and is unique because it lacks a side chain. This gives it more conformational freedom than any other amino acid. Glycine is often found in turns and loops where other amino acids would be sterically unacceptable. Although it is formally nonpolar, it’s very small side chain makes no real contribution to hydrophobic interactions. Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acid serine.
- Abbreviations: Gly, G
- IUPAC name: 2-aminoacetic acid
- Molecular formula: C2H5NO2
- Molecular weight: 75.07 g/mol
glycoside → glikozid
Glycoside is one of a group of organic compounds in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. The sugar group is known as the glycon and the non-sugar group as the aglycon. According to the IUPAC definition, all disaccharides and polysaccharides are glycosides where the aglycone is another sugar.
In the free hemiacetal form, sugars will spontaneously equilibrate between the α and β anomers. However, once the glycosidic bond is formed, the anomeric configuration of the ring is locked as either α or β. Therefore, the alpha and beta glycosides are chemically distinct. They will have different chemical, physical, and biological properties. Many glycosides occur abundantly in plants, especially as flower and fruit pigments.
The term glycoside was later extended to cover not only compounds in which the anomeric hydroxy group is replaced by a group -OR, but also those in which the replacing group is -SR (thioglycosides), -SeR (selenoglycosides), -NR1R2 (N-glycosides), or even -CR1R2R3 (C-glycosides). Thioglycoside and selenoglycoside are legitimate generic terms; however the use of N-glycoside, although widespread in biochemical literature, is improper and not recommended here (glycosylamine is a perfectly acceptable term). C-Glycoside is even less acceptable. All other glycosides are hydrolysable; the C-C bond of C-glycosides is usually not. The use and propagation of names based on C-glycoside terminology is therefore strongly discouraged.
hydrosphere → hidrosfera
Hydrosphere (from the Greek for water sphere) is a discontinuous layer of water on, under, and over the Earth's surface. It includes all liquid and frozen surface waters, groundwater held in soil and rock, and atmospheric water vapour. Water continuously circulates between these reservoirs in what is called the hydrologic cycle, which is driven by energy from the Sun.
| Reservoir | V / 106 km3 | w / % |
|---|---|---|
| oceans | 1 370.0 | 97.25 |
| ice caps and glaciers | 29.0 | 2.05 |
| groundwater | 9.5 | 0.68 |
| lakes, rivers | 0.127 | 0.01 |
| soil moisture | 0.065 | 0.005 |
| atmosphere (as liquid equivalent of water vapour) | 0.013 | 0.001 |
| biosphere | 0.0006 | 0.00004 |
| TOTAL | 1 408.7 | 100 |
luminescence → luminiscencija
Luminescence (from Latin lumen, light) is the emission of electromagnetic radiation (UV, visible or IR) from atoms or molecules as a result of the transition of an electronically excited state to a lower energy state, usually the ground state. Luminescence can be divided into categories by duration (fluorescence or phosphorescence) or by the mechanism that creates the light (radioluminescence, electroluminescence, photoluminescence, thermoluminescence, triboluminescence, chemiluminescence, bioluminescence). The prefix identifies the energy source responsible for generating or releasing the light.
Phosphorescence is emission of light from a substance exposed to radiation and persisting as an afterglow after the source of excitation has been removed. Fluorescence, on the other hand, is an almost instantaneous effect, ending within about 10-8 second after excitation.
mustard agent → plikavac
Mustard agents are usually classified as blistering agents owing to the similarity of the wounds caused by these substances resembling burns and blisters. However, since mustard agents also cause severe damage to the eyes, respiratory system and internal organs, they should preferably be described as blistering and tissue-injuring agents. Normal mustard agent (yperite), 1,1-thio-bis-[2-chloroethane], reacts with a large number of biological molecules. The effect of mustard agent is delayed and the first symptoms do not occur between 2-24 hours after exposure. At room temperature, mustard agent is a liquid with low volatility and is very stable during storage.
nucleic acid → nukleinska kiselina
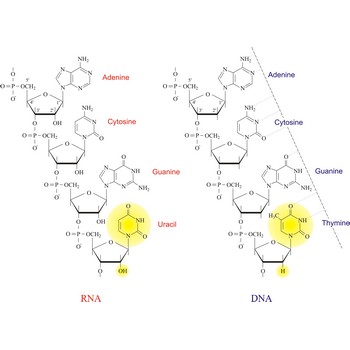
Nucleic acids are a complex, high-molecular-weight biochemical macromolecules composed of nucleotide chains that convey genetic information. The most common nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Each nucleic acid chain is composed of subunits called nucleotides, each containing a sugar, a phosphate group, and nitrogenous base. DNA was first discovered in 1869 by the Swiss biochemist Friedrich Miescher (1844-1895).
Both DNA and RNA contain the two major purine bases adenine (A) and guanine (G) and one of the major pyrimidines, cytosine (C). Of the other two pyrimidines, thymine (T) is found in DNA and uracil (U) is found in RNA. There are two major pentoses in nucleic acids:2'-deoxy-D-ribose in DNA and D-ribose in RNA.
Nucleotides are linked together in both DNA and RNA in a polymeric fashion via covalent bonds. These bonds exist through phosphate-group bridges in which the 5' hydroxyl group of one nucleotide unit is joined to the 3' hydroxyl group of the next nucleotide. RNA is usually a single-stranded molecule, whereas DNA is usually double-stranded.
radioactive indicator → radioaktivni indikator
By use of suitable radioactive isotopes biochemical processes can be observed in plants, animals and humans, by measuring radioactive radiation of radioactive indicator. Artificial radioactive isotopes have the same chemical properties as natural ones, which enable us to mark those natural isotopes with addition of artificial ones and in this way follow the path of those elements during a chemical reaction. One of the most important radioactive indicators is the radioactive carbon 14C.
sedimentary rocks → sedimentne stijene
Sedimentary Rocks are formed by the accumulation and subsequent consolidation of sediments into various types of rock. There are three major types of sedimentary rocks:
Biogenic sedimentary rocks are formed from organic processes when organisms use materials dissolved in water to build a shell or other skeletal structure.
Clastic sedimentary rocks are composed directly of the sediments or fragments from other rocks.
Chemical sedimentary rocks are formed through evaporation of a chemical rich solution.
Based on their sizes, sediment particles are classified, based on their size, into six general categories:
- boulder (>256 mm)
- cobble (64 - 256 mm)
- gravel (2 - 64 mm)
- sand (1/16 - 2 mm)
- silt (1/256 - 1/16 mm)
- clay (<1/256 mm)
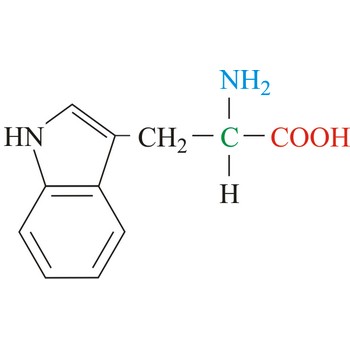
tryptophan → triptofan
Tryptophan is hydrophobic amino acids with aromatic side chain. Tryptophan is large aromatic residue that is normally found buried in the interior of a protein and is important for protein stability. Tryptophan has the largest side chain and is the least common amino acid in proteins. It has spectral properties that make it the best inherent probe for following protein folding and conformational changes associated with biochemical processes. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Trp, W
- IUPAC name: 2-amino-3-(1H-indol-3-yl)propanoic acid
- Molecular formula: C11H12N2O2
- Molecular weight: 204.23 g/mol
Chitosan → Kitozan
Chitosan is a linear polysaccharide composed of randomly distributed N-acetyl D-glucosamine and D-glucosamine units. It can be easily derived from partial deacetylation of natural polymer chitin. At a minimum deacetylization level of 60 % (amount of free amino groups in the polymer) it is considered to be chitosan. Thanks to the amino groups of D-glucosamine, chitosan can be protonated and turned into polycation, which is one of the sources of unique properties of chitosan as biopolymer, like aqueous solubility, antibacterial properties, biodegradability with non-toxic residues and biocompatibility.
Citing this page:
Generalic, Eni. "Biot-Savartov zakon." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table