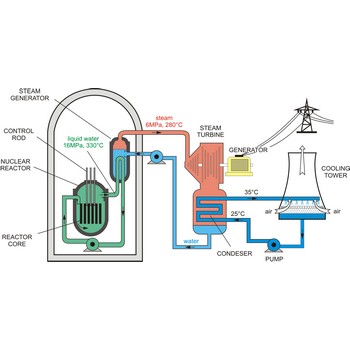
nuclear reactor → nuklearni reaktor
Nuclear reactor is an assembly of fissionable material (uranium-235 or plutonium-239) designed to produce a sustained and controllable chain reaction for the generation of electric power.
The essential components of a nuclear reactor are:
- The core, metal rods containing enough fissionable material to maintain a chain reaction at the necessary power level (as much as 50 t of uranium may be required).
- A source of neutrons to initiate the reaction (such as a mixture of polonium and beryllium)
- A moderator to reduce the energy of fast neutrons for more efficient fission (material such as graphite, beryllium, heavy water, and light water are used)
- A coolant to remove the fission-generated heat (water, sodium, helium, and nitrogen may be used)
- A control system such as rods of boron or cadmium that have high capture cross sections (to absorb neutrons)
- Adequate shielding, remote-control equipment, and appropriate instrumentation are essential for personnel safety and efficient operation.
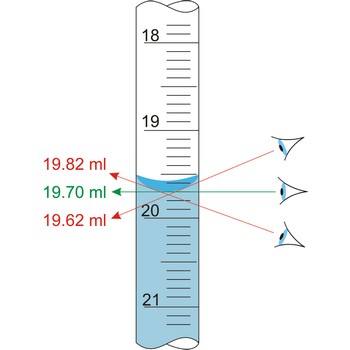
parallax → paralaksa
Parallax is a deceptive change of the position of an object which is observed while the position of the observer changes. Position of eye at all volumetric vessels must be at the same level as the meniscus. If not, the parallax will cause an error while reading the position of the meniscus of a liquid in a burette. It will be a positive mistake if the eye is lower, and negative if the eye is higher than the meniscus plane.
period → perioda
Periods are horizontal rows in the periodic table, each period begin with an alkali metal (one electron in the outermost principal quantum level) and ending with a noble gas (each having eight electrons in the outermost principal quantum level, except for helium, which is limited to two).
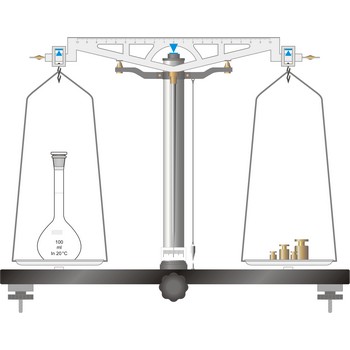
precision balance → tehnička vaga
Precision balances typically display results from three to one decimal places (0.001 g up to 0.1 g). The readability precision balances are reduced when compared to analytical balances but, precision balances accommodate higher capacities (up to several kilograms). In its traditional form, it consists of a pivoted horizontal lever of equal length arms, called the beam, with a weighing pan, also called scale, suspended from each arm.
In electronic top pan, or toploader balances, mass is determined not by mechanical deflection but by electronically controlled compensation of an electric force. The signal generated enables the mass to be read from a digital display. The mass of the empty container can be stored in the balance’s computer memory and automatically deducted from the mass of the container plus its contents.
Schellbach’s burette → Schellbachova bireta
When colourless liquids are used, parallax mistake is avoided by use of Schellbach’s burette. On the inside wall opposite to graduation scale it has a melted in ribbon from milky glass in the middle of which a blue line is found. The level of liquid is now spotted very easily because of light breaking in the meniscus blue line now looks like a double spike.
thermal resistance → toplinski otpor
Heat always flows from a higher to a lower temperature level. The driving force for the heat flux lies in the temperature difference ΔT between two temperature levels. Analogous to Ohm’s law, the following holds:
where H = dQ/dt is heat flux, measured in watts, ΔT is temperature difference across the thermal resistance, measured in kelvin, and Rth is thermal resistance, measured in K/W.
For example, suppose there were two houses with walls of equal thickness; one is made of glass and the other of asbestos. On a cold day, heat would pass through the glass house much faster. The thermal restistance of asbestos is then higher than of glass.
If the thermal Ohm’s law is divided by the heat capacity C, Newton’s law of cooling is obtained:
where dT/dt is rate of cooling or heating, measured in K s-1, and C is heat capacity, measured in J K-1.
thermometer → termometar
Thermometers are devices for measuring temperature. Linear and volume thermal expansion are macroscopic properties of matter, which can be easily measured, relative to measurements of microscopic properties, on the basis of which, temperature is defined. Thermometers based on thermal expansion are secondary instruments that is, they have to be calibrated in comparison to a standard thermometer. In a thermometer with liquid, mercury or alcohol is placed in a small glass container. If temperature increases, the liquid undergoes volume expansion and rises in a capillary. The level of the raised liquid is the measure of temperature. Mercury thermometers measure temperatures in the temperature range between -39 °C and 300 °C. Alcohol thermometers measure lower temperatures. Bimetal thermometers have a spiral spring, which consists of two metals with different coefficients of linear expansion. When temperature changes, metals undergo different change in length and the consequence twisting of the spring is transferred to a pointer, the deflection of which is the measure of temperature.
thermosphere → termosfera
Thermosphere is the layer of the earth’s atmosphere extending from the top of the mesosphere (80 km - 90 km above the surface) to about 500 km. It is characterised by a rapid increase in temperature with increasing altitude up to about 200 km, followed by a levelling off in the 300 km - 500 km region.
Citing this page:
Generalic, Eni. "BIOGRAFIA LEVI MONTALCINI PDF GRATIS." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table