body-centered orthorhombic lattice → prostorno centrirana ortorompska rešetka
Body-centered orthorhombic lattice (orthorhombic-I), like all lattices, has lattice points at the eight corners of the unit cell plus an additional points at the center of the cell. It has unit cell vectors a≠b≠c and interaxial angles α=β=γ=90°.
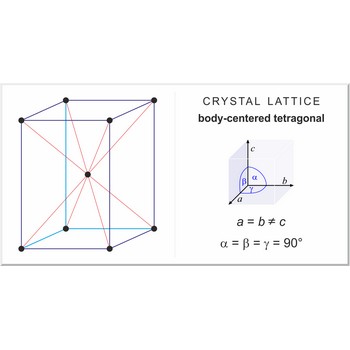
body-centered tetragonal lattice → prostorno centrirana tetragonska rešetka
Body-centered tetragonal lattice (tetragonal-I), like all lattices, has lattice points at the eight corners of the unit cell plus an additional points at the center of the cell. It has unit cell vectors a=b≠c and interaxial angles α=β=γ=90°.
crystal system → kristalni sustav
Crystal system is a method of classifying crystalline substances on the basis of their unit cell. There are seven unique crystal systems. The simplest and most symmetric, the cubic (or isometric) system, has the symmetry of a cube. The other six systems, in order of decreasing symmetry, are hexagonal, tetragonal, rhombohedral (also known as trigonal), orthorhombic, monoclinic and triclinic.
|
Crystal system
|
Unit-cell
|
Conditions on unit-cell edges and angles |
|
cubic |
 |
a=b=c α=β=γ=90° |
|
hexagonal |
 |
a≠c α=γ=90° β=120° |
|
tetragonal |
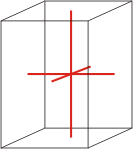 |
a=b≠c α=β=γ=90° |
|
rhombohedral |
 |
a=b=c α=β=γ≠90° |
|
orthorhombic |
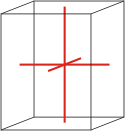 |
a≠b≠c α=β=γ=90° |
|
monoclinic |
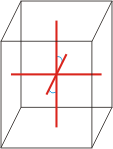 |
a≠b≠c α=γ=90°≠β |
|
triclinic |
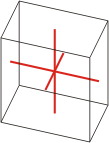 |
a≠b≠c α≠β≠γ≠90° |
cubic crystal system → kubični kristalni sustav
Cubic crystal system is also known as the isometric system. The Isometric crystal system characterizes itself by its three equivalent crystallographic axes perpendicular to each other.
a = b = c
α = β = γ = 90°
electromagnetic radiation spectrum → spektar elektromagnetskog zračenja
Wavelengths of electromagnetic waves can be shown with the help of electromagnetic radiation spectrum. Electromagnetic radiation spectrum is divided into several areas from γ-radiation of very short wavelengths and great energy to radio waves with wavelengths up to 1 000 m. The human eye can only see a narrow part of the electromagnetic spectrum - visible radiation.
face-centered cubic lattice → plošno centrirana kubična rešetka
Face-centered cubic lattice (fcc or cubic-F), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centers of each face of the unit cell. It has unit cell vectors a =b =c and interaxial angles α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the fcc structures the spheres fill 74 % of the volume. The number of atoms in a unit cell is four (8×1/8 + 6×1/2 = 4). There are 26 metals that have the fcc lattice.
face-centered orthorhombic lattice → plošno centrirana ortorompska rešetka
Face-centered orthorhombic lattice (orthorhombic-F), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centers of each face of the unit cell. It has unit cell vectors a≠b≠c and interaxial angles α=β=γ=90°.
hexagonal lattice → heksagonska rešetka
Hexagonal lattice has lattice points at the twelve corners of the hexagonal prism and at the centers of the two hexagonal faces of the unit cell. It has unit cell vectors a=b≠c and interaxial angles α=β=90° and γ=120°.
mass concentration → masena koncentracija
Mass concentration (γ) is equal to mass (mA) of soluted substance and volume (V) of the solution proportion. SI unit for mass concentration is kg m-3, but in laboratory practice g dm-3, which has the same number value, is often used.
monoclinic crystal system → monoklinski kristalni sustav
Minerals of the monoclinic crystal system are referred to three unequal axes. Two of these axes (a and c) are inclined toward each other at an oblique angle; these are usually depicted vertically. The third axis (b) is perpendicular to the other two and is called the ortho axis. The two vertical axes therefore do not intersect one another at right angles, although both are perpendicular to the horizontal axis.
a ≠ b ≠ c
α = γ = 90° ≠ β
Citing this page:
Generalic, Eni. "AI game." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table