electrophoresis → elektroforeza
Electrophoresis is a technique for the analysis and separation of colloids, based on the movement of charged colloidal particles in an electric field. The migration is toward electrodes of charge opposite to that of the particles. The rate of migration of the particles depends on the field, the charge on the particles, and on other factors, such as the size and shape of the particles.
Electrophoresis is important in the study of proteins. The acidity of the solution can be used to control the direction in which a protein moves upon electrophoresis.
electroplating → galvaniziranje
Electroplating (also called electrodeposition) is the deposition of a metallic coating onto an object by putting a negative charge onto the object and immersing it into a solution which contains a salt of the metal to be deposited. The metallic ions of the salt carry a positive charge and are attracted to the part. When they reach it, the negatively charged part provides the electrons to reduce the positively charged ions to metallic form.
Typically, a brass or nickel object is coated with a layer of silver by making use of electrolysis of a silver solution, using the object to be coated as the cathode. The anode consist of pure silver, and the cathode is the object to be plated. The electrolyte is a mixure of silver nitrate with potassium cyanide. The reactions are:
The cyanide ensures a low concentration of silver ions, a condition for providing the best plating results.
neutralisation → neutralizacija
Neutralisation is the process in which an acid reacts with a base to form a salt and water.
energy → energija
Energy (E, U) is the characteristic of a system that enables it to do work. Like work itself, it is measured in joules (J).
The internal energy of a body is the sum of the potential energy and the kinetic energy of its component atoms and molecules.
Potential energy is the energy stored in a body or system as a consequence of its position, shape, or state (this includes gravitation energy, electrical energy, nuclear energy, and chemical energy).
Kinetic energy is the energy of motion and is usually defined as the work that will be done by a body possessing the energy when it is brought to rest. For a body of mass m having a speed v, the kinetic energy is mv2/2. Kinetic energy is most clearly exhibited in gases, in which molecules have much greater freedom of motion than in liquids and solids.
In an isolated system energy can be transferred from one form to another but the total energy of the system remains constant.
epoxy resin → epoksi smola
Epoxy resins are thermosetting resins produced by copolymerising epoxide compounds with phenols (e.g. epichlorohydrin and bisphenol A). They contain ether linkages (-O-) and form a tight, cross-linked polymer network. Toughness, good adhesion, corrosive-chemical resistance, and good dielectric properties characterise epoxy resins. Most epoxy resins are two-part types which harden when blended.
Erlenmeyer flask → Erlenmeyerova tikvica
Erlenmeyer flask is a glass container which has a narrow, cylindrical mouth and a cone-shaped main body that ends in a wide, flat bottom. It was named after its inventor, a German chemist Richard Erlenmeyer (1825-1909). Erlenmeyer flasks are most often used in titrations to hold the liquid that is being titrated.
extraction → ekstrakcija
Extraction is the separation of a component from its mixture by selective solubility. When a solution of one substance in one solvent is brought in with another solvent dissolved substance will distribute between the two solutants because of different solubility. Extraction is an efficient and fast method used for separating and concentrating matters. Extraction is best done several times in a succession, with smaller amount of solvent in it the matter is better dissolved. For example, caffeine can be separated from coffee beans by washing the beans with supercritical fluid carbon dioxide; the caffeine dissolves in the carbon dioxide, but flavour compounds do not. Vanillin can be extracted from vanilla beans by shaking the beans with an organic solvent, like ethanol.
non-Newtonian fluid → nenewtonski fluid
Non-Newtonian fluid is a fluid whose viscosity changes when the gradient in flow speed changes. Colloidal suspensions and polymer solutions like ketchup and starch/water paste are non-Newtonian fluids.
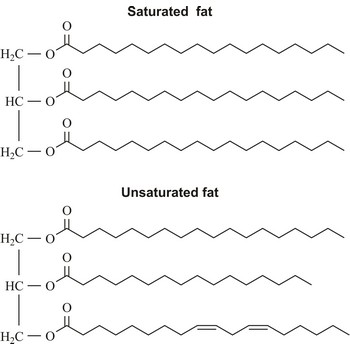
fat → mast
Fats are esters of glycerol and long chain carboxylic acids. Fats occur widely in plants and animals as a means of storing food energy, having twice the calorific value of carbohydrates. Fats derived from plants and fish generally have a greater proportion of unsaturated fatty acids than those from mammals. Fats may be either solid or liquid at room temperature, depending on their structure and composition. Unsaturated fats are liquid at room temperature.
Plant oils may be hardened by the addition of hydrogen atoms, converting double bonds to single bonds. This process is known as hydrogenation. Hydrogenated vegetable oils are often present in margarine and other processed foods.
Alkali hydrolysis of fat with sodium hydroxide it gives glycerol and soap (i.e. a mixture of the sodium salts of the fatty acids).
Citing this page:
Generalic, Eni. "čvrste otopine." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table