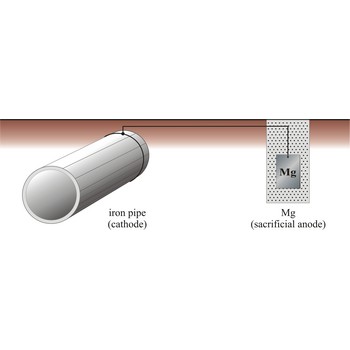
sacrificial protection → zaštita žrtvovanom elektrodom
Sacrificial protection is the protection of iron or steel against corrosion by using a more reactive metal. Pieces of zinc or magnesium alloy are attached to pump bodies and pipes. The protected metal becomes the cathode and does not corrode. The anode corrodes, thereby providing the desired sacrificial protection. These items are known as sacrificial anodes and "attract" the corrosion to them rather than the iron/steel. The sacrificial anodes must be replaced periodically as they corrode.
The iron pipe will be connected to a more reactive metal such as magnesium through cooper wires, the magnesium will donate its electrons to the iron preventing it from rusting. Iron which is oxidises will immediately be reduced back to iron.
cathodic protection → katodna zaštita
Cathodic protection is a process in which a structural metal, such as iron, is protected from corrosion by connecting it to a metal that has a more negative reduction half-cell potential, which now corrodes instead of iron. There are two major variations of the cathodic method of corrosion protection. The first is called the impressed current method, and the other is called the sacrificial anode method.
environment protection → zaštita okoliša
From Environment protection law of the Republic of Croatia: By protection of the environment, the following is ensured: complete preservation of environment quality, natural community preservation, rational usage of natural resources and energy in the most favourable way concerning the environment as a basic condition of healthy and sustainable development.
acylaction reaction → reakcije aciliranja
Acylaction reaction involves the introduction of an acyl group (RCO-) into a compound. An alkyl halide is reacted with an alcohol or a carboxylic acid anhydride e.g.
The introduction of an acetyl group (CH3CO-) is acetylation, a process used for protecting -OH groups in organic synthesis.
aluminium → aluminij
Aluminium was discovered by Friedrich Wöhler (Germany) in 1827. The origin of the name comes from the Latin word alumen meaning alum. It is soft, lightweight, silvery-white metal. Exposed surfaces quickly form protective oxide coating. Metal reacts violently with oxidants. Third most abundant element in the earth’s crust. Aluminium is the most abundant metal to be found in the earth’s crust, but is never found free in nature. Aluminium is obtained by electrolysis from bauxite. Used for many purposes from airplanes to beverage cans. Too soft in its pure form so less than 1 % of silicon or iron is added, which hardens and strengthens it.
anodising → anodizacija
Anodising is a method of coating objects made of aluminium with a protective oxide film, by making them the anode in an electrolytic bath containing an oxidising electrolyte. Anodising can also be used to produce a decorative finish by formation of an oxide layer that can absorb a coloured dye.
beta radiation → beta zračenje
Streams of beta particles are known as beta ray or beta radiation. Beta rays may cause skin burns and are harmful within the body. A thin sheet of metal can afford protection to the skin.
Bordeaux mixture → bordoška juha
Bordeaux mixture is a mixture of copper(II) sulphate and calcium hydroxide in water, used as fungicide.
cadmium coating → kadmiranje
Cadmium coating is a galvanic coating of metallic objects with a cadmium layer for corrosion resistance.
Citing this page:
Generalic, Eni. "Zaštita žrtvovanom elektrodom." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table